Abstract
OBJECTIVE To investigate the effect of polyethylene imine glycol (PEI-PEG)/siRNA nanocomposites in the in vitro transfection of human gastric cancer SGC7901 cell lines and the down-regulation of gene expression of the adherence factor CD44v6.
METHODS PEI-PEG/siRNA nanoparticles, in different N/P ratios, were synthesized and transfected into gastric cancer cells. Lipo2000/siRNA was used in the control group. The transfection efficiencies were observed under fluorescence microscope. The cytotoxicity of the nanoparticles was measured using the MTT assay (mononuclear cell direct cytotoxicity assay), and the down-regulation effect of siRNA on CD44v6 gene was evaluated by Western blot. Based on the different N/P ratios, PEI-PEG/siRNA composites were synthesized and transfected into gastric cancer cells. Lipo2000/siRNA was used in the controls. The transfection efficiency was observed under fluorescence microscope. The cytotoxicity of the nanoparticles was measured using the MTT assay and the down-regulation effect of siRNA on CD44v6 gene was evaluated by Western blot.
RESULTS After transfection, the transfection efficiency of the PEI-PEG/siRNA nanocomposites increased incrementally in N/P ratio value. The transfection efficiency improved with an increase in N/P ratio. When the N/P value was 15, fluorescence became more intense in the PEI-PEG/siRNA group than in the Lipo2000/siRNA group. At the same time, cell viability was (80.4 ± 5.6)% in the MTT reduction assay, which was similar to that in the Lipo2000/siRNA group. The results of Western blot analysis showed that the expression level of CD44v6 protein decreased to (59.7 ± 3.0)% after siRNA-CD44v6 was inhibited.
CONCLUSION PEI-PEG could effectively form the nanocomposite in combination with siRNA, be transfected into the SGC7901 gastric cancer cell lines and inhibit CD44v6 protein expression. Moreover, as a genetic carrier, PEI-PEG copolymer has greater advantages, including high transfection efficiency, less cytotoxicity and an easily alterable vector structure.
keywords
Introduction
CD44 is both a multifunctional cellular adhesion molecule (CAM) and a cell surface receptor. It mainly participates in the binding and adhesion processes among cells as well as between cells and the extracellular matrix. CD44 has several subtypes, including CD44s (standard type), CD44E (epithelial cell type), and CD44v (variant type)[1]. CD44v, especially CD44v6, plays an important role in the distant metastasis of several cancers, such as gastric cancer, and in the infiltration and growth of tumor cells[2].
At present, RNA interference technology, which is considered a highly effective and highly specific approach to the inhibition of gene expression, has been employed in the treatment of many types of diseases such as cancer, infection, and disorders of the respiratory and immune systems[3]. However, putting siRNA into cells requires that the carrier system break through the many barriers that exist both within and outside of cells[4]. Therefore, the carrier system is a key factor in gene therapy. Carrier systems are typically categorized by vector, namely viral vectors and non-viral vectors. Recently, the application of biomedical polymers as new non-viral genetic vectors has demonstrated clinical potential in a wide spectrum of cancer treatments, and a number of advantages have been seen, such as less cytotoxicity and a more easily alterable vector structure.
In our study, polyethylene imine (PEI) glycol (PEI-PEG), a new copolymer, was utilized as a siRNA vector. PEI was attached to the nonionic hydrophilic group, polyethylene glycol (PEG, 2 kDa) to form a PEI-PEG copolymer. The copolymer could not only lower the positive ion toxicity of PEI but could also raise the stability of nanoparticles in the blood, therefore, extending the time that it circulates in the blood[5,6]. Various N/P ratios (e.g. the ratio between the theoretical electric charges loaded by the amino group of copolymer and the phosphate group of siRNA) were used for the synthesis of the PEI-PEG/siRNA nanocomposite and for transfection into the SGC7901 cancer cell lines in vitro. Its role in the gene silencing of gastric cancer cell CD44v6 was observed, and the cytotoxicity and transfection efficiency of the nanocomposites (NaCom) were investigated.
Materials and Methods
Reagents
PEI-PEG was synthesized by the Zhongshan University School of Chemistry and Chemical Engineering Science; PEI (25 kDa) and mPEG-OH (2 kDa) were purchased from Sigma-Aldrich Co.; the SGC7901 cell line was bought from the Institute of Biochemistry and Cell Biology, Shanghai; 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was bought from Sigma-Aldrich Co.; the cell culture reagents were procured from the GIBCO Co.; siRNA was synthesized by the Shanghai JiMa Company; liposome Lipofectamine 2000 (Lipo2000) was obtained from Invitrogen Co.; the primary antibody was purchased from the Bender Co. Synthetic siRNA sequence included siRNA-CD44v6, 5’ -CAG UGG UUU GGC AAC AGA UTT-3’, negative control siRNA, 5’-GCG ACG AUC UGC CUA AGA- 3’, fluorescently-labeled siRNA, and 5’-UUC UCC GAA CGU GUC ACG UTT 3’.
Cell culture
The culture conditions of the human gastric cancer cell lines SGC7901 required: a high-glucose culture medium, DMEM containing a 10% fetal bovine serum; 37°C constant temperature; and incubation in 5% CO2.
Synthesis of the PEI-PEG copolymer
The synthesis of activated mono-methyl ether polyethylene glycol succinate (mPEG2k-NHS), was performed as previously reported by Shuai et al.[7] The procedure for the synthesis of PEI-PEG was as follows: 1.25 g of branching PEI (25 kDa, Aldrich) and 1.0 g of mPEG-NHS were added to a PBS buffer solution (pH 7.4). After overnight agitation at room temperature, the mixture was dialyzed using a bag filter in distilled water for 48 h. Following cryodesiccation, solid PEI-PEG was obtained and stored for later use.
Preparation of PEI-PEG/siRNA NaComs
Deionized water was used to dissolve PEI-PEG copolymer into solutions of various concentrations (0.24-1.42 mg/mL). Based on different N/P ratios (5, 10, 15, 30), the siRNA (20 μM) solution and the PEI-PEG solutions of different concentrations noted above were added to a certain volume of deionized water, maintaining a volume ratio of 1:1.33, until the final volumes of each solution were equal. NaCom of different N/P ratios could be prepared after light vibration, to ensure sufficient mixing, followed by allowing the solution to stand at room temperature for a 10-15 min incubation.
Gene transfection test
At 24 h before transfection, SGC7901 cell lines were placed in 96-well plates at 4 × 104 cells/well. The PEI-PEG/siRNA NaCom was prepared based on different N/P ratios. One hundred pmol of fluorescently-labeled FAM-siRNA and a specified amount of copolymer solution were added separately to 500 μL of PBS solution and then mixed by light vibration. This was followed by allowing the solution to stand at room temperature for a 10-15 min incubation. The original DMEM culture media in the plates was discarded and substituted by new DMEM complete medium. Thereafter, the composites were added to the wells and mixed by light vibration. At the same time, Lipo2000 was used as the control, and the Lipo2000/siRNA composite was synthesized following Lipo2000 specifications. The post-transfected gastric cancer cells were cultured at 37°C for 5 h, and then new culture medium was used. The cells were observed under fluorescence microscope.
MTT test
SGC7901 cell lines were inoculated in 96-well culture plates at 9000 cells/well, at 37°C, overnight. The PEI-PEG/siRNA polymer solution with various N/P values and the Lipo2000/siRNA complex were added separately to the wells. Interventional materials were not added to the control wells, and cells and interventional materials were not added to the blank wells. PEI-PEG/siRNA NaCom was synthesized in each well using 20 pmol siRNA. After incubation at 37°C for 48 h, the used medium was discarded and replaced by serum-free DMEM medium. A 20-μl MTT solution was added in each well (5 mg/mL), and then cultured at 37°C for 4 h. The supernatant was then discarded, and 100-μL DMSO was added to each well followed by a 10-min vibrating incubation. A 492-nm absorbance was detected in the microplate scanning spectrophotometer. The test was repeated 3 times for each well.
Western blot
Seventy-two h after gene transfection, total protein was extracted in RIPA buffer solution (Beyotime, Shanghai). The BCA protein quantitation kit (Shenneng Bocai BioTech Co. Ltd, Shanghai) was used to determine the protein concentration. The GADPH gene was used as the internal reference. The total protein was separated using 10% SDS-PAGE, and then transferred onto a PVDF membrane. The membrane was blocked at room temperature for 2 h in a sealed bag with solution made from 5% skimmed milk powder, followed by a second incubation with the primary antibody (1:1000) at 4°C, overnight. Subsequently, the membrane was rinsed 3 times using TBST buffer solution followed by incubation at room temperature for 1 h using the secondary antibody (1:5000, Jackson, USA). Following this, the membrane was rinsed another 3 times in TBST buffer solution and then exposed to air. Bands were observed. Quantity One software was used to analyze the image.
Statistical analysis
Mean ± SD was used to represent the experimental data. SPSS11.0 software was used for analysis, and one-way ANOVA was used for intergroup comparison. The value of P < 0.05 indicated a statistically significant difference in groups.
Results
Synthesis of PEI-PEG
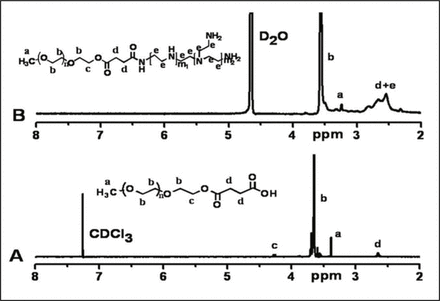
1H-NMR represented a synthesized copolymer, PEI-PEG, which indicated that a proton-chemical shift, i.e. 3.65 ppm and 3.38 ppm, respectively corresponded to a chemical shift on -OCH2CH2- and on CH3O- proton in the PEG chain; and 2.5-2.9 ppm corresponded to a chemical shift on -CH2CH2NH-methylene in the PEI chain (Fig. 1), suggesting that the PEG chain had been successfully linked onto the PEI chain.
NMR spectrum representation of mPEG-COOH (A) and mPEG-PEI (B)1H-NMR spectra of mPEG-COOH (A) and mPEG-PEI (B).
Gene transfection test
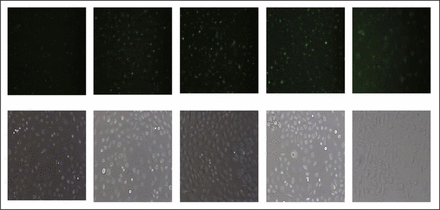
PEI-PEG/siRNA NaCom was synthesized using fluorescently-labeled siRNA, and the fluorescence intensity (FluInt) of the FAM-siRNA that was transfected into the gastric cancer cells was observed under inverted fluorescence microscope. As shown in Fig. 2, the transfection efficiency changed with alterations to the N/P ratios of the NaCom. As the N/P value gradually increased, the level of siRNA that was transfected into the gastric cancer cells also gradually increased. When the N/P ratio was 5, the FluInt of the siRNA in the cancer cells became the weakest. As the N/P ratio increased up to 30, the FluInt increased gradually. When the N/P ratio was 15, the FluInt was similar to that in the Lipo2000/siRNA group.
Image of SGC7901 cell lines by fluorescence microscope (after cell transfection of PEI-PEG/siRNA NaCom and Lipo2000/siRNA with various N/P ratios)
MTT test
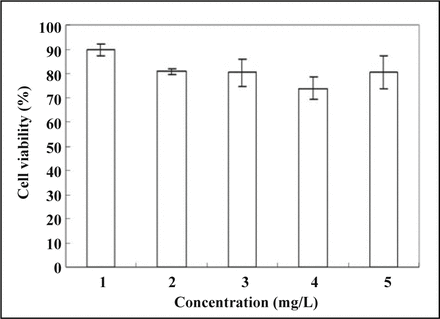
The MTT test was used to determine the toxic effects of the PEI-PEG/siRNA composites in the cells. The wells of the culture plates only with DMEM medium and without any processing were assigned to the blank group, and the cell viability was 100%. The PEI-PEG/siRNA and Lipo2000/siRNA of various N/P ratios were transfected into the SGC7901 cells, with 48 h incubation at 37°C. As indicated in Fig. 3, the cytotoxicity of PEI-PEG/siRNA gradually increased with the increase in N/P ratios. As the N/P ratio approached 15, cell viability reached (80.4 ± 5.6)%, while the cell viability was (80.5 ± 6.7)% in the Lipo2000/siRNA group. The cell viability in each of the 2 groups was similar. When the N/P value was 30, cell viability was (73.8 ± 4.8)% in the transfected PEI-PEG/siRNA group, which was lower than that in the Lipo2000/siRNA group.
Cytotoxicity after transfection of PEI/PEG/siRNA and Lipo2000/siRNA copolymers with different N/P ratios into SGC7901 cells (mean ± standard deviation, n = 3, P < 0.05). The numbers 1, 2, 3 and 4 in Fig. 3 signify the N/P ratios, which in turn represent the PEI-PEG/siRNA composite at the N/P ratio of 5, 10, 15 and 30, respectively; the number 5 represents the Lipo2000/siRNA composites.
Western blot
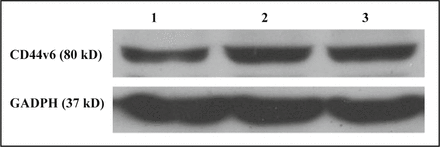
Based on the results of the gene transfection and toxicity tests, the PEI-PEG/siRNA NaCom with an N/P ratio of 15 was selected for transfection into the SGC7901 cells. The role of the siRNA in gene silencing was analyzed by Western blot analysis. As seen in Fig. 4, the band was weaker in the siRNA-CD44v6 group compared with that in the negative controls, showing that siRNA-CD44v6 could inhibit the expression of CD44v6 at the protein level. It was shown by Quantity One analysis that the expression of the CD44v6 protein was reduced to (59.7± 3.0)%.
Results of Western blot analysis following cell transfection of PEI-PEG/siRNA. 1, Group with siRNA-CD44v6/PEI-PEG composite; 2, Negative controls siRNA/PEI-PEG; 3, Group without any treatment.
Discussion
Quite a few authors have believed[8,9] that CD44, especially CD44v6 is related to carcinogenesis, infiltration, and metastasis of cancer. Cancer cells with high expression of CD44v6 can escape monitoring by the immune system which should identify and kill cancer cells. In addition, it is felt that CD44v6 can easily infiltrate into lymph nodes to metastasize to other areas of the body and facilitate tumor invasion into the extracellular matrix, and, as a result, alter the transference and motor ability of tumor cells[10]. Therefore, the application of the RNAi technology to the treatment of gastric cancer to interfere with CD44v6 expression should reduce tumor metastasis, and also play a significant role in the diagnosis and treatment of tumors. Over the past few years, the non-viral vector, with a basis of liposome and cationic polymers, has been commonly used as a carrier in the utilization of siRNA in vitro. Compared to the viral vector, it has advantages, such as low immunogenicity and increased safety[11]. Among cationic polymers, PEI as the genetic carrier has been most frequently used. As a non-viral vector, PEI of various types (branched and linear types) and of different molecular weights (10, 25, 80 kDa) has been successfully applied in basic research. In PEI, the positive charge loaded by the amid group neutralizes the negative charge loaded by the nucleic acid phosphate group to form a stable polymer, i.e. the nucleic acid NaCom. It is well known that the positive charge in NaCom results in better stability of NaCom in blood. NaCom can combine with the negative charge on the cell membrane, thus achieving high transfection efficiency[12]. However, PEI and PEI/nucleic acid composites induce high cytotoxicity[13]. In our study, PEG was used to modify the highly toxic PEI, and they were synthesized as the macromolecular PEI-PEG copolymer. As a genetic carrier, this copolymer can raise the genetic transfection efficiency and lower cytotoxicity[13-15]. It can also reduce the cytotoxicity and the size of nanoparticles by optimizing the proportion between the 2 polymers.
Our study revealed that when the value of the N/P ratio was between 5 and 30, the FluInt became intensive with an increase in N/P ratio, i.e., more siRNA could be transfected into the gastric cancer cells. Nevertheless, compared to the condition with the N/P ratio of 15, there was no apparent increase in transfection efficiency in the 2 groups as the N/P ratio reached 30. The reason may be related to the fact that the higher the N/P ratio reaches, the more intensively the siRNA and PEI-PEG are compressed together; therefore, over-compression results in siRNA being difficult to release[16]. It can be seen in Fig. 2 that the transfection efficiency in the PEI-PEG/siRNA group at an N/P ratio of 15 is higher than that in the Lipo2000/siRNA group, suggesting that PEI-PEG can effectively transfer siRNA into gastric cancer cells. This is due to the fact that PEI-PEG loads positive charge and when it follows siRNA to form a composite, the composite also loads positive charge. The larger the value of the N/P ratio, the more positive charge the composite loads, resulting in an ready combination of PEI-PEG and the cell membrane loading negative charge to transport the exogenous genes into cells.
At present, Lipo2000 has been used as a genetic carrier and is widely utilized in some research on RNA interference. However, it has been proven in various studies that Lipo2000 induces high cytotoxicity[17,18]. In our study, when the N/P ratio was less than 15, the cytotoxicity of PEI-PEG/siRNA was lower than that of Lipo2000/siRNA. Therefore, compared with Lipo2000, PEI-PEG as a non-viral vector has shown greater advantages, such as increased safety, higher transfection efficiency, easier full scale production, higher stability, and a more easily alterables structure. According to the results of gene transfection and cytotoxicity tests, PEI-PEG/siRNA with an N/P ratio of 15 has been used for transfection into SGC7901 cell lines. The results of Western blot analysis have shown that when PEI-PEG is used as the carrier, there is down-regulation in the expression of siRNA-CD44v6 on the CD44v6 gene in the gastric cancer cell lines. Our study has demonstrated that PEI-PEG can transport siRNA into the SGC7901 cells and can effectively release siRNA so as to elicit gene-silencing effects.
In this study, a nanometer polymer was used to transport siRNA into SGC7901 cell lines and to inhibit the expression of CD44v6 in gastric cancer cells. Compared with Lipo2000, PEI-PEG has multiple advantages including low toxicity, high trasfection efficiency, low cost, and an easily alterable vector structure. Therefore, PEI-PEG will be one of the prospective genetic carriers in research on gene therapy for gastric cancer.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Footnotes
This work was supported by the following funds: National Natural Science Foundation of China (No.30670951); Guangdong Provincial; Natural Science Foundation (No.06021322); Fund of Guangzhou Municipal; Scientific Problem-Solving Program (No. 2003 Z 3-E0381); Fund of Guangdong; Provincial Scientific Problem-Solving Program (No.2005 B31211002); Guangdong Provincial Government and Ministry of Education Project combining project initiation, study and research (No.2009B090300277).
- Received March 4, 2010.
- Accepted June 9, 2010.
- Copyright © 2010 by Tianjin Medical University Cancer Institute & Hospital and Springer