Abstract
OBJECTIVE To compare the differences of mitochondrial DNA (mtDNA) copies among the tissues of esophageal squamous cell carcinoma (ESCC), para-neoplastic tissue and normal mucous membrane of the esophagus, and to study the relationship between the mtDNA and the occurrence and development of esophageal squamous cell carcinoma.
METHODS The mtDNA copies of 42 specimens with the ESCC, paraneoplastic mucous tissue and normal mucous membrane of the esophagus were determined using real-time fluorescence quantitative PCR. The mtDNA was analyzed using agarose gel electrophoresis.
RESULTS The mtDNA from all of the tissues (42/42) from the ESCC, para-neoplastic tissue and normal esophageal mucous membranes was analyzed, showing that there were an average mtDNA copy number of 27.1894×106 μg DNA, 9.4102×106 μg DNA and 5.9347×106 μg DNA, from the respective tissues. There were significant differences (F=27.83, P<0.05) in mtDNA copy number among the three. A positive band was shown at 403 bp after gel electrophoresis of the PCR products, and the lane where the ESCC mtDNA located was rather bright, which was in accordance with the result of the real-time PCR determination.
CONCLUSION An increase in the mtDNA copy number is related to the occurrence and development of ESCC.
keywords
INTRODUCTION
Studies have indicated that the extrachromosomal DNA might take part in tumorigenesis. Significant increases in the mitochondrial DNA (mtDNA) content, lack of mtDNA, mutations[1] and changes of the microsatellite instability (MSI)[2] have been shown to exist in a variety of tumor cells. Mutations of the mitochondrial DNA and abnormalities of the mitochondrial structure and function might relate to many kinds of oncogenesis[3], but a report of a study on relationship between a change in the number of the mtDNA copies and onset and progress of the ESCC has not as yet been published. In our study, fluorescent quantitative PCR (i.e. the real-time PCR) was used to quantitatively assay the mtDNA copies in ESCC tissue, para-neoplastic tissue and normal mucous membranes of the esophagus, in order to offer clues for further investigation on the correlation between the mtDNA copies and onset and development of ESCC.
MATERIALS AND METHODS
Tissues
The tissues of 42 cases with ESCC were all obtained from surgical specimens of the resection performed in the Anyang Cancer Hospital, Anyang, Henan, China, during the period from February 16 to March 26, 2006. All were pathologically confirmed as ESCC. The ESCC tissue, para-neoplastic mucous tissue and normal esophageal mucous membrane was studied from each case.
Methods
Extraction of mtDNA
A 100 mg sample of each tissue was used to extract the DNA using the procedures from a mtDNA extraction kit and a mtDNA isolation kit (supplied by the Shanghai Genmed Science Co. Ltd.).
Design and synthesis of the primers
The mtDNA PCR primers was designed and synthetized by the Shanghai Genmed Science Co. Ltd. The upstream primer was 5′-CAC CCT AGC CAT CAT TCT AC-3′ and the downstream primer 5′-GGG GTA TGC TGT TCG AAT TC-3′. The length was 403 bp.
Preparation of the standard curve
The mtDNA was extracted from 100 mg of tissues to prepare 8 standard preparations (The concentration of the mtDNA was 1×1010, 1×109, 1×108, 1×107, 1×106, 1×105, 1×104 and 1×103, respectively). Then a 0.5 μl reaction template, 0.5 μl upstream primer, 0.5 μl downstream primer, 15 μl reaction liquid, 1.25 μl fluorochrome and 7.25 μl balanced solution were combined to prepare a 25 μl fluorescent quantitative PCR reaction system. The procedure was completed based on the following parameters: 95°C for 2 min; 95°C 30 s, 59°C 1 min, 72°C 30 s, 35 cycles and 72°C for 5 min.
Determination of the mtDNA copies
The real-time PCR was used to detect the number of mtDNA copies in the three tissue types from the 42 specimens. The conditions for the reactions were the same as those used for preparation of the standard curve.
Quantitative determination of real-time PCR products
The real-time PCR products were quantified by using agarose gel (15 g/L) electrophoresis (AGE), with a voltage of 60 V and a time of 20 min.
Statistical analysis
The SPSS10.0 software was used for statistical analysis of all the data. The analysis of variance (ANOVA) was employed to determine the differences in the mtDNA copies among the ESCC, paraneoplastic mucosal and normal esophageal mucosal tissues. The q test was utilized to compare difference in the mtDNA copies in three tissue types. The significant level was: α=0.05.
RESULTS
Real-time PCR standard curve
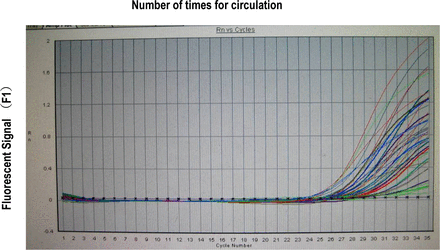
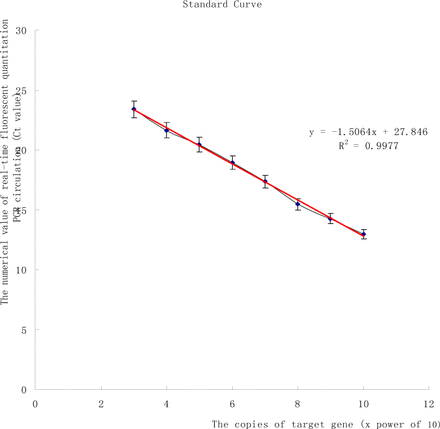
The fluorescent signal was obtained by a real-time PCR analysis. The number of times for the circulation corresponding to the inflection point entering the exponential growth stage from the background at the beginning was the Ct value (Fig. 1). The mtDNA copy number of the specimen was acquired after comparison of the Ct value with the standard curve (Fig. 2). The mtDNA copies in every microgram of DNA was calculated based on the template DNA concentration.
The real-time PCR dynamic determination of the level of 126 mtDNA samples.
Standard curve drawn based on 8 standard preparations with a known concentration.
For comparisons of the mtDNA levels between the ESCC, paraneoplastic mucosal and normal esophageal mucosal tissues see Table 1.
Statistical table for the mtDNA copies in the ESCC, paraneoplastic mucosal and normal esophageal mucosal tissues.
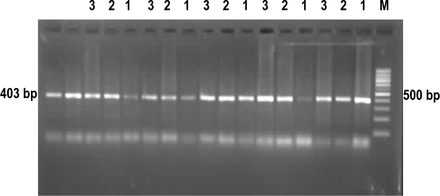
Results of the gel electrophoresis of the PCR products
The results of the gel electrophoresis in Fig. 3 showed that a positive band of mtDNA occurred in the three tissue types at the 403 bp site. Moreover, the bands of the DNA from the cancerous tissues were brighter than those from the paraneoplastic mucosal tissue and, those from the paraneoplastic mucosa were brighter than those from the normal esophageal mucosa.
The results of the electrophoretic assay of real-time PCR products. M: 1000 bp ladder marker, 1, normal esophageal mucosa; 2, paraneoplastic mucosa; 3, ESCC.
DISCUSSION
Over the past few years, studies on the relationship between mtDNA and tumors have begun in some institutions in China and overseas, and some hypotheses have been developed. The available data have suggested that the tumor biological features not only depend on the intranuclear genetic material but also on the characteristics of the tumor extranuclear DNA[4]. Tumorigenesis might be related to the fact that mtDNA is prone to damage and is not easily repaired. The mtDNA genome has weak repair systems and is directly exposed to hyperoxia during oxidative phosphorylation, which is a major factor for the damage. In addition, the structure of the mitochondrial genome is as follows: a) the ratio of lipid/DNA in mitochondria is high, which allows lipophilic cancinogens to preferentially accumulate in the mitochodria; this can account for only part of the total DNA damage in cells[5]. b) mtDNA lacks protein protection of the nuclear genome, i.e., it is bare. Therefore, mtDNA is an important carcinogic target, and its degree of injury and mutation rate is markedly higher compared to the nuclear DNA (approximately 10 times or more)[6]. Owing to poor development and low efficiency of the mtDNA repair mechanism, mutations in the mtDNA may cause functional mitochondrial defects. Only excess copies of the mtDNA can compensate for the defects[7].
Vijay et al.[8] have estimated mtDNA levels in malignant and benign cells, using a digoxin-marked mtDNA probe in situ. The results showed that there was a manifest increase in mtDNA copies in the malignant cells. Even though the biological significance is not as yet clear, the reason for this difference may relate to a loss of control of the mtDNA copying mechanism. From our point of view, early research[8] on mtDNA structure showed no credible difference in the overall structure, length, density of the mtDNA molecules, constituent ratio of the nucleotides and the cyclic dimmer, etc. between cancer cells, benign tumor cells and normal cells. Boutwood, et al.[9] applied a mtDNA probing method of in situ hybridization using peripheral blood or bone marrow cells, and found that, the mtDNA copies of all patients with acute leukemia and most of the patients with chronic leukemia (8/9) had increased significantly. The study of Luciakova, et al.[10] showed that there was an increase in energy requirement of the tumor cells, but whether or not the increase in the mtDNA copies was an adaptation reaction to provide the needs for tumor cell growth was not clear. It has been shown that the mtDNA copies in gastric cancer were decreased[11]. According to the findings, because of the ROS damage to the mtDNA or to the D-loop area, it prolonged the replicative cycle of mtDNA, diminution of the copy number or enhanced destruction of the mtDNA.
It was found in our quantitative study of the mtDNA in esophageal cancers, that there was a progressive increase in the mtDNA copy number from the tissues of normal esophageal to paraneoplastic mucosa, to ESCC. Furthermore, there was a overt difference in the order of magnitude and also a significant difference in the mutual comparison between the three tissue types (P<0.05), suggesting a close correlation between the increase of the mtDNA copies and the occurrence and progress of ESCC. These results provide significant clues for future research on the biological feature of ESCC tumor cells. Further studies on the importance of these relationships will be forthcoming.
Footnotes
The work was supported by the grand from Key Subjects Construction of the 10th Five-Year Plans 211 Project of the Ministry of Education [No. Jiaozhongban (2002) No.2].
- Received December 10, 2006.
- Accepted January 5, 2007.
- Copyright © 2007 by Tianjin Medical University Cancer Institute & Hospital and Springer