Abstract
OBJECTIVE To investigate whether nimesulide can suppress tumor growth and induce apoptosis in SGC-7901 gastric cancer cells and to explore the molecular mechanism Involved.
METHODS SGC-7901 cells were cultured in RPMI 1640 medium containing different concentrations of nimesulide (0,12.5, 50, 100, 200, 400 μmol/L). The MTT assay, morphological observation, electron microscopy (EM), Immunohistochemical analysis and Western blot analysis were employed to investigate the effects of nimesulide on the SGC -7901 cells and to explore possible related molecular mechanisms.
RESULTS Nimesulide inhibited the growth of SGC-7901 cells and elicited typical apoptotic morphologic changes. Nimesulide also decreased NF-κB and Bcl-2 expression, but Increased the level of the Bax protein. The positive rate of Bel-2 protein expression at 0, 50, 100 and 200 umol/L of nimesulide was 58.3±14.0%, 50.2±9.9%, 32.8±5.0% and 22.7± 5.5% respectively based on immunohistochemical staining. The positive rate of Bax protein expression was 22.0±5.7%, 29.2±6.5%, 42.7±5.9% and 74.5±9.1% and the NF-κB expression was 74.2±10.9%, 61.8±7.6%, 36.7 ±10.9% and 17.5 ±12.3%, Significant differences were found between 0 μmol/L and 100 pmol/L and 200 pmol/L. Western blot analysis also showed that the expression of NF-κB was decreased.
CONCLUSION Nimesulide suppresses tumor growth and induces apoptosis by inhibiting NF-κB expression, which may be related to the overexpression of Bax relative to Bcl-2 expression.
keywords
ABBREVIATIONS:N F-κB is a nuclear transcription factor that regulates expression of a large number of genes involved in the regulation of apoptosis, viral replication, tumorigenesis, inflammation, and many autoimmune diseases.[1] NF-κB is activated by a variety of stimuli that include growth factors, cytokines, lymphokines, radiation, pharmacologic agents, and stress. Use of a NF-κB inhibitor (kinase inhibitor, PS-1, 145) can block the protective effect of IL-6 against apoptosis induced by both conventional (dexamethasone) and novel (immunomodulatory derivative of thalidomide, IMÍD3) therapies.[2]
It is known that the continuous activation of NF-κB may pose a risk, i.e. cells with a pro-survival phenotype may give rise to continuously proliferating cells and thus may be tumorigenic.[3] Furthermore it is known that a new defined class of proto-oncogenes contribute to malignancy by inhibiting apoptosis. Previous studies have shown that alterations in the ratio between pro-apoptotic and anti-apoptotic members of the Bcl-2 family, rather than the absolute expression level of any single Bcl-2 family member, can determine apoptotic sensitivity. Therefore, it appears to be the relative ratios of Bax and Bcl-2 that determine the fate of a cell, rather than the absolute level of either protein.[4]
Nonsteroidal anti-inflammatory drugs (NSAIDs), such as non-selective cyclooxygenase aspirin [5] and sulindac,[6] may play a role in the inhibition of proliferation and induction of apoptosis of tumor cells through the inhibition of NF-κB activity. Celecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor, is a non-steroidal anti-inflammatory drug which has at this time been approved by the USA FDA for adjuvant treatment elf patients with familial adenomatous polyposis.[7] Other selective COX-2 inhibitors have anti-tumor actions. At the present time it is not known in what way the NF-κB pathway is related to the action of selective cyclooxygenase drugs. In addition the literature related to the apoptotic balance in the gastro-intestinal tract following treatment with selective COX-2 inhibitors is still scarce and conflicting. So the present study was designed to determine whether nimesulide, a selective cyclooxygenase inhibitor can suppress tumor growth and induce apoptosis in SGC-7901 gastric cancer cells and to explore the molecular mechanism involved.
MATERIALS AND METHODS
Chemicals and reagents
Nimesulide and MTT were obtained from the Sigma Chemical Co. Ltd. Nimesulide was dissolved in DM-SO prior to use. Anti-NF-κB (mouse monoclonal anti-body, sc-8008), anti-Bcl-2 (rabbit polyclonal anti-body, sc-492) and anti-Bax (rabbit polyclonal anti-body, sc-526) monoclonal antibodies were purchased from Santa Cruz Biotechnology, Inc. RPMI 1640 medium was obtained from Gibco Chemical Co. Ltd.
Cell culture
The human gastric cancer cell line, SGC-7901, was purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences and maintained in RPMI 1640 medium supplemented with 10% FCS, 1% glutamine, and 1% antibiotic solution. Cell cultures were grown in 5% C02 with 100% humidity. After 24 h in RPMI 1640 medium, when the SGC-7901 cells adhered to the glass coverslips in the culture plates or at the bottom of the culture plates, the cells were treated with various concentration of nimesulide in RPMI 1640 containing 10% FCS.
MTT assay
The SGC-7901 cells were plated at 1×104 cells/well in 96-well plates in RPMI 1640 containing 10% FCS. After 24 h, the culture medium was replaced by a fresh medium containing the different concentrations of nimesulide (0, 12.5, 50, 100, 200, 400 μmol/L). Twelve duplicate wells were set up for each sample. The cells not treated with the drugs served as negative controls and the cells treated with C-DDP (10 μ,g/ml) served as a positive control. After 24, 48, or 72 h of incubation, 20 |xl MTT (5 g/L) was added to each well and incubation continued for 4 h. The supernatants were then removed, 150 p.1 DMSO was added, followed by shaking for 10 min until the crystals were dissolved and then absorbance measured at 492 nm. The wells without cells were used as the zero point of absorbance. The inhibitory rate (IR) was calculated as follows: IR= (1 —A value of nimesulide well/A value of control well) ×l00%.
Morphological observation
After treatment with nimesulide, cytologic morphological changes were observed under an Olympus optical microscope. Cells were subcultured on coverslips in 6-well culture plates. After 12, 24, 36,48 and 72 h the coverslips were taken out and observed after staining with H&E.
Immunohistochemical analysis
Cells treated with different concentrations of nime-sulide (0, 50, 100, 200 μmol/L) were cultured on coverslips in a 6-well plate. After 48 h the glass coverslips on which the cells were mounted were collected and fixed with 10% neutral formalin for immunohistochemical analysis. The PowerVision™ two-step method was used for the immunostaining of NF-κB, Bcl-2 and Bax. In brief, after washing with PBS, endogenous peroxidase activity was eliminated by preincubation in 3% H202 for 10 min. Primary antibodies were used with the following dilutions: NF-κB (1: 100), Bcl-2 (1:75), Bax (1:50). After incubation overnight at 4°C, the cells were washed with PBS and then incubated with a secondary antibody for 30 min at room temperature. DAB was used as the chromogen and the cells were counterstained with hematoxylin. Negative controls were established by replacing the primary antibody with PBS and known immunostaining-positive slides were used as positive controls. Specific staining for each protein was categorized as either positive or negative based on the presence of brown-color staining. Clear staining of the cytoplasm (NF-κB, Bcl-2 and Bax) was the criterion for a positive reaction. The positive number of cells in each high-power field of 10 microscopic fields were counted and the percentage of positive cells relative to the total tumor cells calcalated as follows: PR=(number of positive cells/total number) xl00%. All the immunostained slides were observed by 2 pathologists.
Western-blot assay
Cells were collected after treatment with different concentrations of nimesulide (0, 50, 100, 200 (μmol/L) for 48 h. Aliquots of cell lysates containing equal protein were separated on SDS-polyacrylamide gels for 2~3 h, and then transferred to nitrocellulose filters followed by incubation with NF-κB antibody (mouse monoclonal antibody, sc-8008, dilution 1:1000) overnight at 4 °C. Then the filters were washed with PBS-T followed by the addition of horseradish peroxidase-linked goat anti-mouse IgG for 2 h at room temperature and NBT/BCIP visualization of the bands.
Transmission electron microscopy
After 48 h when the SGC-7901 cells had been treated with various concentrations of nimesulide (0, 50, 100, 200 μmol/L) in RPMI 1640 containing 10% FCS, the cells were collected and fixed with 4% glutaraldehyde and immersed in Epon 821. The cells were transfered into ultrathin sections (60 nm) and stained with uranyl acetate and lead citrate. Cell morphology was observed by transmission electron microscopy.
Statistical analysis
The PR and A value data are presented as the means ± SD and were compared using one-way ANOVA employing STATA 8.0 software. P<0.05 was considered statistically significant.
RESULTS
Proliferation of SGC-7901 cells
Cell growth was determined by the MTT assay. As shown in Table 1, nimesulide showed a potent inhibitory effect on the growth of the SGC-7901 cells. Additon of nimesulide caused a dose and time-depen-dent inhibitory effect.
Inhibitory effect of nimesulide on SGC-7901 cells (mean±SD, n=12)
Morphological changes
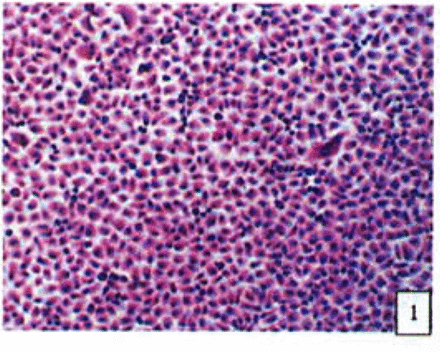
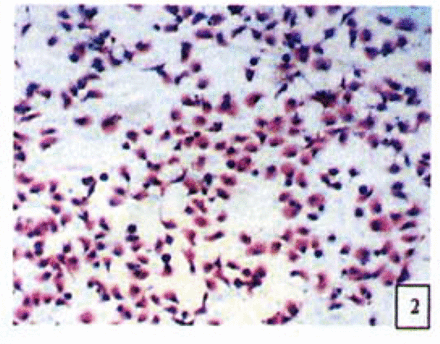
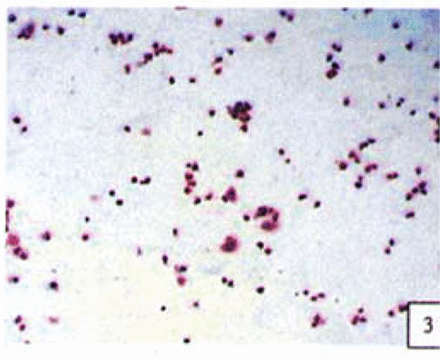
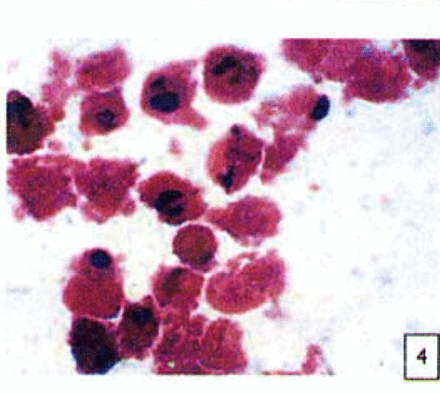
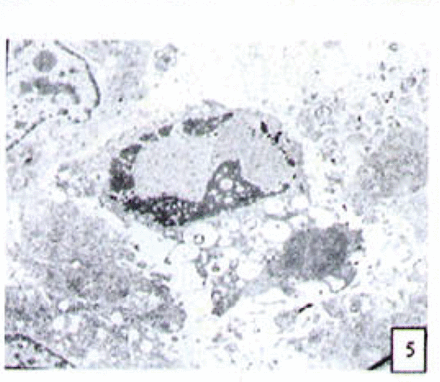
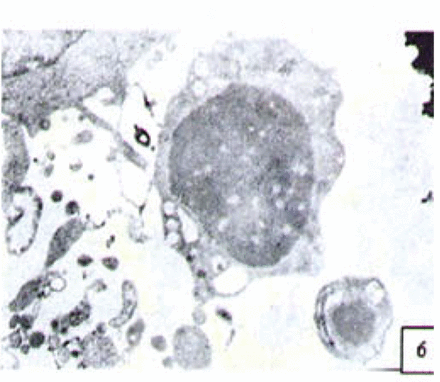
H&E stained cells in the negative control groups showed normal structures Fig. 1, but with increasing levels of nimesulide, the SGC-7901 cells became sparse Fig. 23 and at high concentrations of nimesulide, some dead cells showed apoptotic morphologic changes, such as nuclear condension, fragmentation and chromatin crescents Fig. 4. Under electron microscopy cells at high concentrations of nimesulide also had apoptotic characteristics including chromatin condensation, nuclear fragmentation, chromatin crescents Fig. 5 and apoptotic bodies Fig. 6.
Cells from negative control groups (H&E 10x × 10).
Cells treated with 100,200 p.mol/L nimesulide; with increased concentrations the cells became sparse (H&E 10 × 10).
Cells treated with 100,200 p.mol/L nimesulide; with increased concentrations the cells became sparse (H&E 10 × 10).
Cells treated with 100 |xmol/L nimesulide; cells showed apoptotic morphologic changes, such as nuclear condenstion, fragmentation and chromatin crescents(arrow) (H&E 10 × 100).
Cells treated with 100 p,mol/L nimesulide; under electron microscopy the cells showed apoptotic characteristics including chromatin crescents (EM* 5000 ) and apoptotic bodies (EM × 6000).
Cells treated with 100 p,mol/L nimesulide; under electron microscopy the cells showed apoptotic character × 6000).
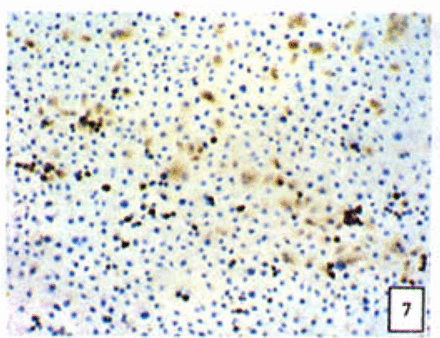
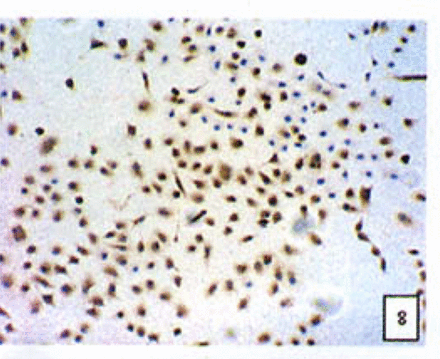
Expression of the Bcl-2, Bax and NF-κB proteins
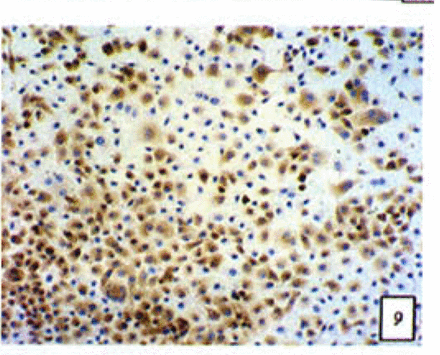
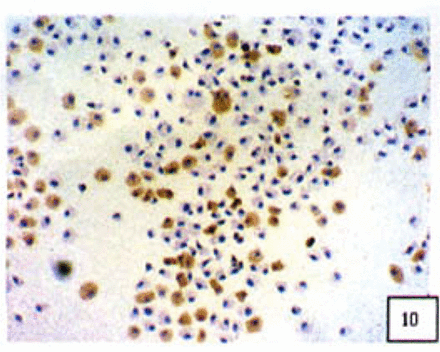
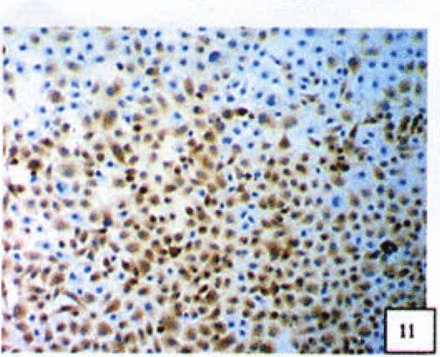
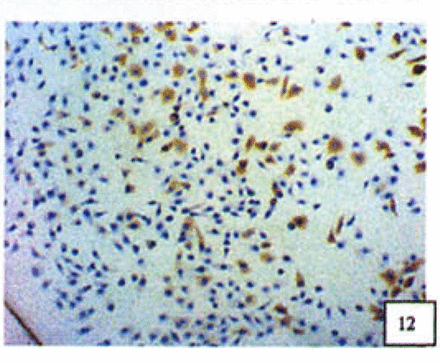
Positive staining was located in the cytoplasm. Fig. 7 to Fig. 12 show the expression of Bcl-2, Bax and NF-κB proteins before and after treatment of nimesulide. With increasing concentrations of nimesulide, NF-κB and Bcl-2 expression was decreased, while the level of the Bax protein was increased. There were significant differences between 0 μmol/L and 100 μmol/L and 200 pjnol/L (P<0.01, Table 2). After 48 h of treatment with nimesulide at different concentrations, Western blot analysis showed that the protein expression of NF-κB was obviously decreased Fig. 13.
Positive rate of Bel-2, Bax and NF-κB protein expression in SGC-7901 cells (n=6)
Expression of Bax cells from the negative control group (PowerVIsion TM two-step method 10* 10 was used for Figs.7-12)
Expression of Bax In cells treated with 200 p,mol/L nimesulide.
Expression of Bcl-2 in cells from the negative control group.
Expression of Bcl-2 in cells treated with 200 p,mol/L nimesulide.
Expression of NF-κB in cells from the negative control group.
Expression of NF-κB in cells treated with 200 |xmol/L nimesulide.
Expression of NF-κB In cells treated with 0,50,100,200 p.mol/L nimesulide (Western-blot assay).
DISCUSSION
NF-κB, originally identified and named for its role in the regulation of immunoglobulin kappa-chain gene expression in B cells, is a dimer composed of p50 (NF-κB 1), p52 (NF-κB 2), c-Rel, RelB, or p65/RelA subunits. In its inactive form, NF-κB is sequestered in the cytoplasm, bound to inhibitor proteins of the IKB family. The various stimuli that activate NF-κB cause phosphorylation of IKB, which is followed by its degradation. This results in exposure of the nuclear localization signals on NF-κB subunits and the subsequent translocation of the molecule to the nucleus. In the nucleus, NF-κB binds with the consensus sequence of various genes and thus activates their transcription. In processes such as tumor initiation and promotion where prolonged survival of cells is a crucial event, NF-κB plays an important role as a mediator of inhibition of apoptosis. NF-κB also modulates the expression of factors responsible for growth as well as apoptosis. Alternatively, the inhibition of NF-κB has resulted in significant anti-tumor effects in melanoma.[8]
Previous studies have demonstrated that NF-κB can inhibit apoptosis not only through regulating expression of anti-apoptotic proteins such as an inhibitor of apoptosis (IAP) proteins c-IAPl and C-IAP2 and other cytokines such as IL-6 and IL-8, but also through up-regulateing the expressiong of Bcl-2. [9] Pro-apoptotic members of the Bcl-2 family such as Bax contribute to the formation of pores in the outer mitochondrial membranes, facilitating leakage of pro-apoptotic proteins into the cytoplasm. Anti-apoptotic members of the Bcl-2 family such as Bcl-2 antagonize the pro-apoptotic members, thus inhibiting the formation of pores. The balance between pro and anti-apoptotic Bcl-2 family members within a cell determines its relative resistance or sensitivity to apoptosis.
A shift in this balance towards a more anti-apoptotic phenotype may result in transformation of a normal cell into a continuous proliferating malignant tumour cell. Some members of the Bcl-2 family, including anti-apoptotic Bcl-2 and Bcl-Xl are under the control of the transcription factor NF-κB. [10] NF-κB can regulate gene transcription and the expression of the Bcl-2 anti-apoptotic protein because Bcl-2 promoter analysis showed multiple putative NF-κB binding sites. [11] Bcl-2 expression was also up-regulated by ectopic p21WAFl[12] and TNF-a.[13] Inhibition of NF-κB activity decreased the expression of gene products in anti-apoptotic involved Bcl-2 protein levels.[14],[15]
Of importance in this study was the finding that with increasing concentrations of nimesulide, the SGC-7901 cells were inhibited in a time and dose-dependent manner. Morphological studies showed that the cells became sparse with apoptotic morphologic changes, such as nuclear condension and fragmentation. These findings were likewise confirmed by electron microscopy demonstrating cellular apoptotic characteristics including chromatin condensation, chromatin crescents, nuclear fragmentation and apoptotic bodies. Another observation in this study was that with increasing concentrations of nimesulide, the expression of NF-κB and Bcl-2 were decreased while that of Bax was increased, so the Bcl-2/Bax ratio was gradually decreased.
Considering the role of NF-κB, Bcl-2 and Bax in apoptosis, we can conclude that nimesulide suppresses tumor growth and induces apoptosis by inhibiting NF-κB expression, which may be related to over-expression of Bax relative to Bcl-2 expression. Multiple studies have shown that NF-κB is involved in the regulation of apoptosis, angiogenesis, and tumor cell invasion, all of which indicate the important role of NF-κB in tumorigenesis. And at the same time, the use of NF-κB inhibitors has resulted in significant anti-tumor effects, so NF-κB may be a molecular target for tumor therapy. This possibility should be followed-up with further comprehensive studies.
- Received December 10, 2005.
- Accepted April 6, 2006.
- Copyright © 2006 by Tianjin Medical University Cancer Institute & Hospital and Springer