Abstract
Monoclonal antibodies (MAbs) are a relatively new innovation in cancer treatment. At present, some monoclonal antibodies have increased the efficacy of the treatment of certain tumors with acceptable safety profiles. When monoclonal antibodies enter the body and attach to cancer cells, they function in several different ways: first, they can trigger the immune system to attack and kill that cancer cell; second, they can block the growth signals; third, they can prevent the formation of new blood vessels. Some naked MAbs such as rituximab can be directed to attach to the surface of cancer cells and make them easier for the immune system to find and destroy. The ability to produce antibodies with limited immunogenicity has led to the production and testing of a host of agents, several of which have demonstrated clinically important antitumor activity and have received U.S. Food & Drug Administration (FDA) approval as cancer treatments. To reduce the immunogenicity of murine antibodies, murine molecules are engineered to remove the immunogenic content and to increase their immunologic efficiency. Radiolabeled antibodies composed of antibodies conjugated to radionuclides show efficacy in non-Hodgkin’s lymphoma. Antivascular endothelial growth factor (VEGF) antibodies such as bevacizumab intercept the VEGF signal of tumors, thereby stopping them from connecting with their targets and blocking tumor growth. Trifunctional antibodies have revealed a new perspective in cancer therapy extending beyond primary destruction of tumor cells.
keywords
Introduction
Monoclonal antibody (mAb) therapy is the use of monoclonal antibodies to bind specifically to target cells or antigens. This may then stimulate the patient’s immune system to attack those cells or inhibit tumor growth. The development of molecular biology techniques promotes research in the field of cancer therapy. Advances in isolating, defining, and measuring appropriate targets for targeted therapies have led to the production of monoclonal antibodies against molecules, which orchestrate pathophysiologic mechanisms for cancer genesis[1]. Thus, a large amount of research and investigations are currently underway to create mAbs for numerous serious diseases (such as rheumatoid arthritis, multiple sclerosis, and different types of cancers)[2,3]. Several general strategies involving mAbs can be used to treat cancer. mAb therapy can be used to destroy malignant tumor cells by activating the immune system and prevent tumor growth by blocking specific cell receptors. In this report, recent advances in antibody-based cancer therapies are reviewed and discussed. Perspectives on antibody-based therapy in the future are also discussed.
Mechanisms of Action for Antibodies as Therapeutic Agents
Antibodies may exert their antitumor effects via a variety of mechanisms of action: (1) by activating the immune system to destroy cancer cells; (2) by interfering with the binding of cytokines or other proteins that are critical for cancer cells to maintain their uncontrolled growth; and (3) by stopping new blood vessels from forming[4]. Several monoclonal antibodies that have been widely used in cancer therapy are described in the following text.
The immune system defends the body by attacking foreign invaders that enter our body from the outside, but it does not always recognize cancer cells inside as enemies. Monoclonal antibodies can bind to the cancer cells having the tumor antigens on their surface and make the cancer cells more detectable to the immune system[5]. Then, the immune system finds the cancer cells marked with bound antibodies as foreign and destroys them. Rituximab (Rituxan) is a humanized monoclonal antibody used to treat non-Hodgkin’s lymphoma, chronic lymphocytic leukemia (CLL), rheumatoid arthritis, and so on. Rituxan selectively binds to CD20, a protein found only on the surface of B cells, which plays an important functional role in B cell activation, proliferation, and differentiation. The mechanisms for rituximab-mediated cell destruction include direct signaling of apoptosis, complement activation, and cell-mediated cytotoxicity[6,7]. Rituximab is thought to attach to the CD20 receptor and initiate intracellular apoptotic control signal pathways. For example, some data from patients with CLL proved that rituximab-mediated apoptosis is caused by caspase-3 activation, and in the Epstein–Barr virus–positive 2F7 cells derived from AIDS-associated Burkitt’s lymphoma, rituximab binding downregulates bcl-2 expression and increases sensitization to chemotherapy acting via mitochondrial pathways. The another postulated mechanism of action of monoclonal anti-CD20 is complement activation through the Fc portion of the antibody, leading to cell lysis or complement-dependent cytotoxicity (CDC). In addition, the in vivo studies from two groups have indicated antibody-dependent cellular cytotoxicity (ADCC) as an important mechanism for the action of rituximab[6,7]. Antibody-induced ADCC needs two binding domains. One domain targets a tumor antigen and the other targets the immunoglobulin Fc receptor on immune effector cells[4]. Therefore, effector cells of the immune system actively lyse target cells that have been bound by specific antibodies. The typical ADCC is mediated by natural killer cells (NK cells), which have an Fc receptor and can recognize the Fc portion of an antibody. NK cell cytotoxicity and cytokine secretion in response to a rituximab-coated B cell is determined by the balance of activating and inhibiting signals through human leukocyte antigen–restricted receptors. Inhibitory signals are via the immunoreceptor tyrosine-based inhibition motif recruiting SHP-1 and activation is via an adapter DAP12 that contains the immunoreceptor tyrosine-based activation motif ITAM[6]. Ofatumumab is another antibody against the CD20 antigen. It is used mainly to treat CLL when other treatments are no longer effective. Alemtuzumab targets CD52, a protein present on both B cells and T cells, and it is used to treat some patients with B-cell CLL[8].
Compared with healthy cells, certain cancer cells frequently make extra copies of the growth factor receptors, making them grow and proliferate faster. Therefore, some antibodies target receptors whose ligands are growth factors, such as the epidermal growth factor (EGF) receptor[9]. The antibody thus inhibits natural ligands that stimulate cell growth from binding to targeted tumor cells[10]. As a result, this may slow or stop the cancer cells from dividing and multiplying. For example, cetuximab blocks HER1, a receptor for EGF that is found on some breast cancers and lymphomas[11]. Herceptin interferes with HER2 receptor, a growth factor receptor overexpressed in one-third of breast cancers.
In addition, antibodies that target components of the tumor microenvironment have been developed, which perturb vital structures such as the formation of tumor-associated vasculature. Cancer cells and healthy cells rely on blood vessels to bring them the oxygen and nutrients they need to grow[12]. Cancer cells send out growth signals to attract blood vessels, which is a key factor in tumor growth. Monoclonal antibodies that block these growth signals may help prevent a tumor from developing a blood supply. In the case of a tumor with an already-established network of blood vessels, blocking the growth signals could cause the blood vessels to die and the tumor to shrink[13]. For example, bevacizumab can bind to vascular endothelial growth factor (VEGF), thereby prevent it from binding to its receptor; it was approved by the U.S. Food & Drug Administration (FDA) in 2004 for the treatment of colorectal cancers[14].
Anti-cancer monoclonal antibodies can be used to target malignant cells through several other mechanisms, such as antibody-directed enzyme prodrug therapy (ADEPT) and immunoliposomes. ADEPT has demonstrated feasibility as a treatment for cancer. In ADEPT, an antibody directed against a tumor-associated antigen is linked to an enzyme, which selectively activates a relatively nontoxic prodrug into a potent cytotoxic agent. Two phases are mainly involved in ADEPT. First, an antibody with the enzyme attached is given, and the enzyme-antibody conjugate results in selective accumulation of the enzyme in the tumor. Then, the prodrug is dosed systemically, which is selectively converted into an active drug by the cancer cells and the drug eventually destroys them[15]. Another attempt to achieve active targeting using high-affinity binding of antibodies to the target is the immunoliposome. Immunoliposome uses antibodies as targeting ligands and lipid vesicles as carriers for both hydrophobic and hydrophilic drugs. At present, two kinds of approach have been considered: the antibodies are conjugated either directly to the lipid bilayer of liposomes or to the distal end of a poly(ethylene glycol) chain[16]. The specific delivery of drugs to the target cells is far more efficient with immunoliposomes than with liposomes lacking antibodies. Drug targeting with immunoliposomes is highly complex and its therapeutic efficacy is influenced by various parameters, such as antigen density on the target cells, the rate of release drug, and the lipid composition of the liposomes.
The Present Antibody Therapeutic Agents
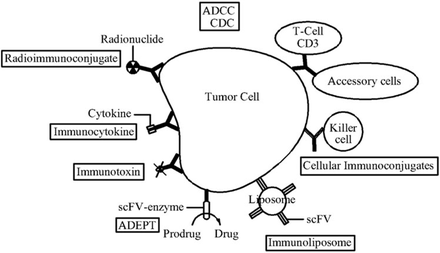
The ability to identify therapeutic targets and produce antibodies with limited immunogenicity has led to the production and testing of a host of agents, several of which have demonstrated clinically important antitumor activity and have been approved by the U.S. FDA for human use. We focus here on some monoclonal antibodies with proven efficacy, classified according to the functions or antigen system that is targeted, and induced into human medicine. Fig.1 shows monoclonal antibodies for cancer therapy.
Monoclonal antibodies for cancer therapy.
Lower immunogenicity antibodies
One immediate goal of antibody engineering is reducing the immunogenicity of murine antibodies, and murine molecules have been engineered to remove immunogenic content and to increase their immunologic efficiency. This was initially achieved through the production of chimeric antibodies. Human/mouse chimeric antibodies have been created in which antibody variable region sequences from the mouse genome are combined with antibody constant region sequences from the human genome[17]. The resulting molecule is approximately 60% of human origin. The chimeric antibodies exhibit the binding characteristics of the parental mouse antibody, and the effector functions associated with the human constant region. This reduces immunogenicity, thereby increasing serum half-life.
Continued efforts to improve monoclonal antibodies led to the development of humanized monoclonal antibodies. Humanized antibodies combines only the amino acids responsible for making the antigen-binding site (the hypervariable regions) of a mouse antibody with the rest of a human antibody molecule, thereby replacing its own hypervariable regions[18]. This results in a molecule of approximately 95% human origin. Humanized antibodies exhibit even less immunogenicity than chimeric monoclonal antibodies. However, several studies have proven that humanized antibodies bind antigen much more weakly than the original murine monoclonal antibodies. According to the report, the affinity represents a several hundredfold decrease[19, 20]. To improve the strength of antigen–antibody interaction, mutations were introduced into the complementarity determining regions (CDR) using techniques such as randomization of CDR, chain shuffling, and so on.
Aside from chimeric and humanized antibodies, human monoclonal antibodies are necessary. Two successful approaches, namely, transgenic mice and phage display libraries have been identified to produce more human monoclonal antibodies[21]. Human monoclonal antibodies were generated using transgenic mice. Human immunoglobulin genes were transferred into the mice genome, and then the transgenic mouse are vaccinated against the desired antigen, leading to the production human monoclonal antibodies. The immunogenicity of fully human monoclonal antibodies is low because they are 100% human and contain no mouse protein. One of the most successful commercial organizations behind therapeutic monoclonal antibodies is Cambridge Antibody Technology (CAT). Phage display has been demonstrated for use such that variable antibody domains could be expressed in filamentous phage antibodies.
Radiolabeled antibodies
By combining a radioactive particle with a monoclonal antibody, radiation can be delivered directly to the cancer cells[22]. Thus, radiolabeled monoclonal antibodies can kill the tumor cells without harming most of the surrounding healthy cells. Radiation-linked monoclonal antibodies deliver a relatively low level of radiation over a longer period of time, which has almost the same effect as the routine high-dose external beam radiation. A variety of radiolabeled anti-CD20 monoclonal antibodies, such as Ibritumomab (Zevalin) and Tositumomab (Bexxar), have been approved for treating the B-cell non-Hodgkin’s lymphoma. Zevalin combines a monoclonal antibody with either the radioactive isotope indium-111 (111In) or the radioactive isotope yttrium-90 (90Y). Zevalin attaches to the receptors on cancerous blood cells and delivers radioactivity directly to cancerous B lymphocytes[23]. Bexxar is a conjugate of a monoclonal antibody and the radioactive isotope iodine-131 (131I), which is used to treat certain types of non-Hodgkin’s lymphoma that no longer respond to rituximab or chemotherapy. Although both Bexxar and Zevalin kill normal B cells, they do not harm the B-cell precursors because these do not express CD20. Therefore, in time, the precursors can repopulate the body with healthy B cells.
Anti-VEGF antibodies
Angiogenesis performs a critical role in the development of cancer. All tumor need to rely on the growth of new blood vessels to bring oxygen and nutrients and remove metabolic wastes. Without this new blood supply, tumors can only grow into 1 to 2 cubic millimeters and not spread. Therefore, anti-angiogenesis is an effective therapeutic strategy for treating cancer. Vascular endothelial growth factor (VEGF) is a key cytokine in the development of normal blood vessels, as well as the development of vessels in tumors and other tissues undergoing abnormal angiogenesis. VEGF also plays an essential role in reproduction and bone angiogenesis. Substantial evidence also implicates VEGF as a mediator of pathologic angiogenesis[24,25]. The two high affinity VEGF receptors, the tyrosine kinases Flt-1 (VEGFR-1) and Flk-1/KDR (VEGFR-2), can deliver growth signals to blood vessels. Anti-VEGF antibodies compete with VEGF receptors for the binding region and block VEGF-induced angiogenesis to inhibit the tumor growth. In clinical trials and as FDA-approved therapeutics, anti-VEGF monoclonal antibodies have demonstrated therapeutic utility in blocking the growth of new blood vessels[26]. For example, the monoclonal antibody bevacizumab (Avastin) was approved in 2004 to treat a number of metastatic cancers such as colorectal and non–small cell lung cancer. Avastin combined with chemotherapy significantly prolongs the survival of related patients[27, 28]. Ranibizumab is a monoclonal antibody fragment derived from the same parent mouse antibody as Avastin and it works by blocking abnormal blood vessel growth and leakage in the eyes to treat wet age-related macular degeneration (AMD).
Trifunctional antibodies
Trifunctional antibodies (trAbs) are characterized by a unique ability to bind three different types of cells: tumor cells, T cells, and accessory cells. It has binding sites for two different antigens, leading to the simultaneous recognition of two cell types, namely, tumor cells and T cells. In addition, its intact Fc domain binds to an Fc receptor on accessory cells and activates them (macrophages, dendritic cells, natural killer cells)[29]. Therefore, trAbs recruit both T cells, as well as accessory cells, such as macrophages, dendritic cells, and natural killer cells, to the tumor site with cancer-associated surface antigens. The subsequent activation of both arms of the immune system, the adaptive one with cytotoxic T cells as effectors and the innate one including accessory effector cells, results in a new high quality of cancer cell killing. Therefore, trAbs are very effective in killing cancer cells and show a therapeutic effect at very low doses. Its efficacy is dependent on the presence of immune effector cells, which confirms the importance of local immunostimulatory effects (e.g. cytokine release and physiologic T cell activation and proliferation) and their contribution to anti-tumor activity. Catumaxomab is a typical trAb. It has two different antigen-binding sites directed at epithelial cell adhesion molecule (EpCAM) and the T-cell antigen CD3[30]. Furthermore, catumaxomab activates Fcγ-receptor I-, IIa-, and III-positive accessory cells via its functional Fc domain. Consequently, catumaxomab is able to redirect T lymphocytes and accessory cells to epithelial cell adhesion molecule-positive tumor cells[31,32]. Then, a complex antitumor immune response is triggered, which leads to the unrestricted but specific killing of epithelial tumor cells[33]. Catumaxomab, which was approved in the EU in April 2009, is the first trAb to receive regulatory approval and the first drug in the world approved specifically for the treatment of malignant ascites. In 2010, it was awarded the Galen von Pergamon Prize[29]. Ertumaxomab is an intact trAb that targets HER2/neu and CD3 with preferential binding to activating Fcγ type I/III-receptors. Ertumaxomab is currently under Phase II studies for treating metastatic breast cancer patients.
Perspectives for Future Research
Monoclonal antibodies are now established as a key therapeutic modality for many types of cancers. Monoclonal antibodies have demonstrated their ability to improve survival rates and the quality of life in some targeted cancers, such as breast cancer, metastatic colorectal cancer, and B cell derived malignancies. Antibodies achieve their therapeutic effect mainly through activation of host defense molecules, by sending apoptotic or growth inhibitory signals, or by blocking angiogenesis[34]. The development of modern recombinant techniques and progressive modification of early murine monoclonal antibodies to reduce and eventually eliminate the mouse protein content led to the sequential development of chimeric, humanized, and totally human antibodies with reduced immunogenicity[35]. There is growing evidence that immunoconjugates such as radiolabeled antibodies are efficacious with acceptable toxicity. Highly active and specific immunoconjugates will improve our diagnostic and therapeutic levels, compared with unconjugated monoclonal antibodies. TrAbs have revealed a new perspective in cancer therapy, which have extended beyond the primary destruction of tumor cells and have increased the efficacy of treatment of certain tumors with acceptable safety profiles. Some MAbs are in phase II and phase III clinical studies and will be submitted to the US Food and Drug Administration for approval in the next few years. Based on recent discoveries in molecular cellular biology and advances in MAbs, we can confidently predict that progress toward more efficacious and less toxic therapy for human cancer in our near future.
Conflict of Interest Statement
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant nos. 30800561 and 31171303) and Tianjin Natural Science Foundation (Grant no. 09JCZDJC18100).
- Received September 8, 2011.
- Accepted October 27, 2011.
- Copyright © 2011 by Tianjin Medical University Cancer Institute & Hospital and Springer