Abstract
Objective To observe the expression of thrombospondin-1 (TSP-1) in prostate cancer, and examine its expression in relation to angiogenesis.
Methods The expression of TSP-1 and microvessel density (MVD) were studied in 22 prostate cancer patients by using immunohistochemistry.
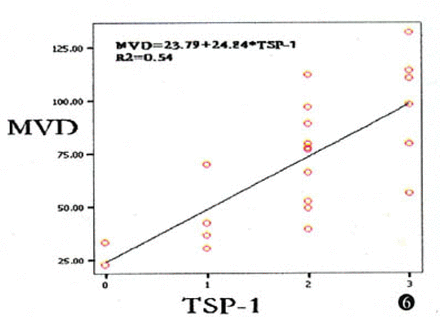
Results Positive expression of the TSP-1 protein was detected in 16 (72.7%) of the 22 cases. Most of the positive staining for TSP-1 was seen in the cytoplasm of the cancer cells, but some was in the extracellular matrix. The mean MVD in the 22 prostate cancer cases was 71.21 ± 31.14 vessels per 100 high field of vision. Tumors with an elevated expression of TSP-1 showed a high MVD resulting in a correlation between TSP-1 immunopositivity and microvessel density that was highly significant (r=0.54, P=0.009).
Conclusion TSP-1 is strongly expressed in most prostate cancers and is associated with neovascularization. Therefore TSP -1 is a likely contributor to the extensive neovascularization in prostate cancer and increased TSP-1 expression might participate in an angiogenic phenotype.
keywords
Neovascularization is a type of biological behavior of solid tumors that plays an important role in the production of metastasis. It is known that tumor progression closely correlates with angiogenesis which depends on the local balance between many factors that induce or inhibit neovascularization. The purpose of the present study was to analyze the level of TSP-1 expression in 22 prostate cancer and to correlate those findings with MVD.
Materials and Methods
Tissue samples
A total of 22 prostate cancers from October 1999 to May 2004 were studied, including 9 radial prostectomy speimens and 13 prostatic puncture speimens. The mean age of the patients was 65.7±6.9 years. Tumors were staged according to the UICC staging system of 1997. Of the 22 cases there were 2 in stage I (T1N0M0 2), 7 in stage II (T2N0M0 6, T1N1M0 1), 3 in stage III (T3N1M0 3) and 10 in stage IV (T4N1M1 4, T4NxM1 6)(Table 1).
Analysis of immunostaining
Immunohistochemical staining was performed using an immunoperoxidase SABC Kit (Boster, Wuhan, China). The sections were respectively incubated with anti-CD34 antibody (Santa Cruz Corp, USA; dilution 1:100) and anti-TSP-1 antibody (Ab-1, NeoMarkers Corp, USA; dilution 1:100) overnight at 4°C, followed by incubation with the secondary antibody for 30 min. The immunocomplexes were visualized using DAB (Zhongshan Biosciences, Beijing, China).
The cases were classified as follows: negative (−), no stained cells present; weak (+), <10% of the entire population of cells stained; moderate (++), >10% and <50% of the entire population of cells stained; and strong (+++), >50% of the population stained moderate to strong. In order to statistically analyze the data, the negative group included both negative and weak detections and the positive group included both moderate and strong results.
The microvessel density (MVD) was determined based on CD34-positive tubular structures. Employing a procedure based on the method described by Takahashi et al.[1] To measure the intra-tumor MVD, the stained sections were screened without any knowledge of the patients clinical data at × 40 magnification to identify the highest vascular area within the tumor. At these highly vascular areas, the individual microvessel count was evaluated at × 100 magnification. The mean MVD obtained from 3 fields was used for analysis.
Statistical analysis
Statistical analyses were performed using the χ2 test, the Fisher’s exact test and Student t-test. P values < 0.05 were regarded as statistically significant.
Results
Microvessel density
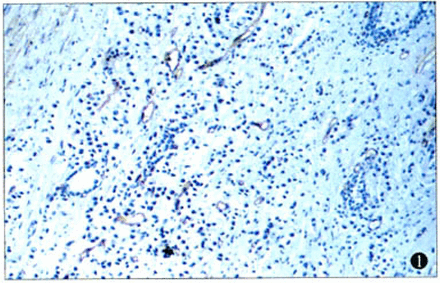
MVD was evaluated using CD34-positive structures as an indiator in the 22 prostate cancer cases, The distribution of the CD34-positive endothelial cells was heterogeneous and aggregated on the cancer periphery. The overall mean MVD in the highest vascular area was 71.21 ±31.14 vessels per 100 high-power field. In 8 cases with lymph node metastasis (the microvessel counts 81.85 ±21.63) were significantly higher, compared to 8 cases without lymph node metastasis (52.19±25.15, P=0.024). Furthermore, the microvessel counts in 10 cases with distal metastasis (88.74±28.47) were significantly higher than those in 12 cases without distal metastasis (56.6±26.0, P=0.012, Fig.1, Table 2).
Immunohistochemical staining of microvessel (CD34) in prostate cancer (× 100).
TSP-1 protein expression and clinicopathological factors
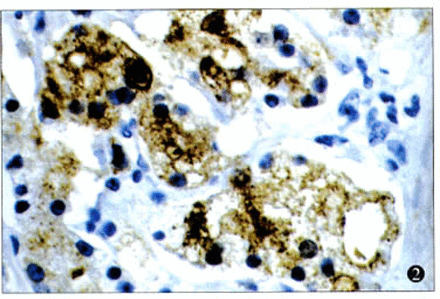
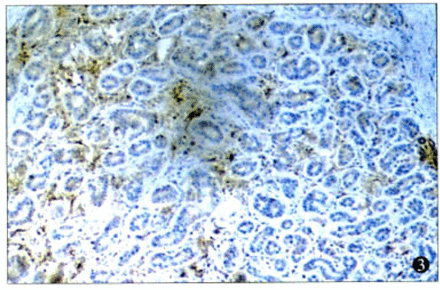
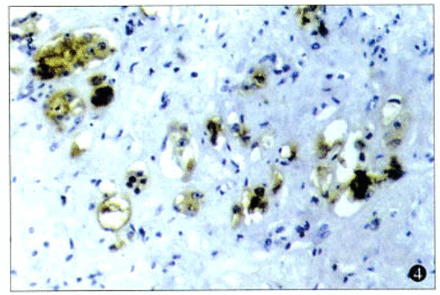
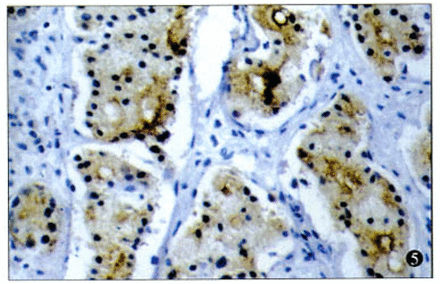
There were 72.7% (16/22) of the cases with strong positive staining for TSP-1. In addition, positive staining for TSP-1 was seen in the cytoplasm of the cancer cells (Fig.2), but some staining was found in the extracellular matrix (Fig.3). While there were 2 types of patterns in the TSP-1 cytoplasm staining—diffuse immunoreactivity in the cytoplasm (Fig.4) and dot-like immunoreactivity in peri-nuclei(Fig.5)—these 2 patterns did not show any difference relative to the clinicopathological stage, histological type, and lymphnode or distal metastasis.
Immunostaining of TSP-1 in the cytoplasm of the prostate cancer cells (× 400).
Immunostaining of TSP-1 in the extracellular matrix of prostate cancer (× 100).
Dot-like immunostaining of TSP-1 in the cytoplasm (× 200).
Diffuse immunostaining of TSP-1 in the cytoplasm (× 200).
Correlation between TSP-1 and MVD
In the 22 prostate cancer cases, tumors in the high MVD group showed a higher incidence of TSP-1 expression (100%) compared to those in the low MVD group (45.45%), the correlation between TSP-1 expression and MVD being statistically significant (r= 0.54, P=0.009, Fig.6).
Correlation between MVD and TSP-1 expression (r=0.54, P=0.009).
Discussion
Solid tumors require neovascularization for tumor growth, progression and metastasis. Evaluation of angiogenic factors involved in the mediation of neovascularization is of important, because this information can afford novel opportunities for therapeutics. TSP-1 has been shown to play a role in angiogenesis, but presently the role of TSP-1 in tumor neovascularization and progression is controversial. In the present study, we investigated the expression and distribution of TSP-1 in prostate cancers and analyzed the correlation between TSP-1 and MVD, as well as the correlation between TSP-1 and lymph node metastasis, and TSP-1 and distal metastasis.
TSP-1, a 45kDa multifunctional platelet and extracellular matrix glycoprotein, is one member of the TSP-1 family, which plays an important role in modulation of proliferation, metastasis and angiogenesis.[2] In the present study, a high level of TSP-1 expression was detected in 72.7% of prostate cancers. TSP-1 was mostly expressed in the cytoplasm of cancer cells, but some was expressed in the tumor stromal tissues. These results are consistent with some other previous reports. It has been noted that TSP-1 was expressed in the cytoplasm of thyroid, breast and prostate cancer cells,[3-5] but some reports showed TSP-1 expression only in stromal tissues of certain cancers such as those of the pancreas and colorectum.[6,7] Therefore, the pattern of TSP-1 expression in a tumor may differ depending on the organ involved or histological type.
In our study, we found a highly significant positive correlation between TSP-1 expression and intra-tumor MVD in the prostate cancers (P=0.009). Both Rice et al.[8] and Miyanaga et al.[9] showed that TSP-1 expression was inversely correlated with MVD in breast tumors and colorectum tumors, in contrast to Zhang et al.[10] who reported that expression of TSP-1 coincided in gastric carcinoma with a high microvessel density. Similar to our findings, Kasper et al.[9] showed that elevated TSP-1 expression was positively associated with higher MVD in pancreas cancer. These studies overall suggest the effects of TSP in tumor angiogenesis may be dependent on the tumor type and environmental setting. However, there is growing evidence that the effect of TSP-1 in angiogenesis is concentration dependent. At low concentrations, TSP-1 inhibits angiogenesis, whereas at high concentrations, it is stimulated.[11] Taraboletti et al.[12] demonstrated the formation of two fragments (25 kDa and 140 kDa) exerting opposing actions on vascular endothelial cells following enzymatic proteolysis of the TSP-1 molecule. The heparin-binding 25-kDa fragment is the angiogenic domain of TSP-1, while the 140-kDa fragment retains the angio-suppressive effect.[13]
In conclusion, TSP-1 was strongly expressed in the cytoplasm of the prostate cancers and the level of expression was positively associated with neovascularization. These findings indicate that TSP-1 might participate in the mediation of neovascularization in prostate cancer and thereby be involved in the tumor angiogenic phenotype.
Footnotes
This work was supported by the National Natural Science Foundation of China (No.30471731).
- Received November 20, 2005.
- Accepted January 3, 2006.
- Copyright © 2006 by Tianjin Medical University Cancer Institute & Hospital and Springer