Abstract
OBJECTIVE Breast-conserving surgery has been a standard treatment for relatively small size of breast cancer. Younger breast cancer patients have more desire to conserve their breasts. This study was to investigate the clinicopathological characteristics and prognosis of younger breast cancer patients who received breast-conserving treatment in China.
METHODS The data of 232 breast cancer patients who received breast-conserving treatment in Cancer Hospital of Chinese Academy of Medical Science from January 1st, 1999 to December 31st, 2005, were collected and retrospectively analyzed. According to the age, the patients were divided into 2 groups: younger group (age ≤ 35 at the time of diagnosis) and elder group (age > 35). The clinical features of the patients in the 2 groups were compared, and their clinical characteristics, recurrence, metastasis and survival status were summarized.
RESULTS In the 232 cases, younger patients accounted for 15.9% (37/232), the elder 84.1% (195/232). By December 2008, the median time of follow-up was 54 months (ranging from 2 months to 118 months). Two patients (5.41%, 2/37) in the younger group and 5 patients (2.56%, 5/195) in the elder group died. The 5-year overall survival rate (OS) in the younger and elder groups was 96.08% and 97.19%, respectively (χ2 = 0.69, P = 0.4066). Local recurrence (LR) or distant metastasis (DM) presented in 5 patients (5/37, 13.51%) in younger group. LR or DM presented in 10 patients (10/195, 5.13%) in elder group. The 5-year disease-free survival (DFS) rate in the younger and elder groups was 82.58% and 95.52%, respectively (χ2 = 4.02, P = 0.0451). Lymph node status and the age of 35 years old or younger were the prognosis factors affecting the DFS of patients who received breast-conserving treatment (OR = 3.467, 95%CI: 1.048-11.472, P < 0.05; OR = 0.245, 95%CI: 0.069-0.863, P < 0.05). Lymph node status was the only prognostic factor affecting the DFS of younger group patients (OR = 7.357, 95%CI: 1.030-52.563, P < 0.05).
CONCLUSION Though the younger and elder patients have the same mid-long term survival rate, younger patients are more likely to have recurrence or metastasis than the elder patients. Breast-conserving surgery given to the younger patients especially to the younger patients with lymph nodes positive should be contemplated cautiously.
keywords
Introduction
Breast-conserving treatment (BCT) is the choice of therapy for relatively small size of breast cancer, and usually makes decent cosmetic outcomes. Some randomized clinical studies have confirmed that the long term survival rate among patients who have relatively small size of breast cancer and undergo breast-conserving treatment is the same as that among patients who undergo radical mastectomy[1-3]. For cosmetic reasons, young women (age 35 or younger at diagnosis) with breast cancer are inclined to BCT.
The incidence of breast cancer in young women is low, while the disease has been traditionally considered as particularly aggressive and associated with a poor prognosis. Usually, breast cancer in young women are poorly differentiated, of which estrogen receptor (ER) and progesterone receptor (PR) are more likely to be negative[4], but human epidermal growth factor receptor 2(HER-2) is positive[5]. However, there are some studies providing the results different from those. The positive rate of hormonal receptors in young patients with breast cancer is the same as that in older patients[5].
The aim of the present study was to retrospectively investigate the clinicopathological characteristics and prognosis of younger breast cancer patients who received breast-conserving treatment in China, and to evaluate the outcome of breast-conserving treatment in younger breast cancer patients.
Patients and Methods
Clinical data
The study population consisted of 232 women with breast cancer, who were diagnosed and treated with BCT in Cancer Institute/Hospital, Chinese Academy of Medical Science between 1999 and 2005. Patient records were reviewed. And all the breast-conserving patients in this period were enrolled. The patients were divided into 2 groups: younger group (age 35 or younger at the time of diagnosis) and elder group (elder than 35 years old at the time of diagnosis). Thirty seven of the 232 patients were identified into younger group, and 195 into elder group.
The mean age of the patients was 44.91 years (range, 23.0-83.0 years). Most of these patients presented with a self-discovered, palpable breast mass and subsequently underwent the examinations of mammograms, ultrasound and biopsy with fine or core needle. All the masses were located in the periphery tissue of the breast, and the distance between nipple-areolar region and the edge of the tumor was 3 cm at least. No enlarged lymph nodes were found in the ipsilateral axillary fossa by physical examination and ipsilateral multifocal or bilateral breast cancer was excluded with imaging. The clinical stage was T1 in 171 patients (76.68%) and T2 in 52 patients (23.32%). The tumor stage of 9 patients who received lumpectomy for biopsy in other hospitals was Tx because of inadequate information. Pathologic characteristics were recorded from the analysis of the original pathologist, and the infiltrating ductal carcinoma was found predominantly in 199 patients (85.78%), infiltrating lobular carcinoma in 6 (2.59%), ductal carcinoma in situ or lobular carcinoma in situ in 6 (2.59%), medullary carcinoma in 9 (3.88%), other histological types in 12 (5.16%). ER protein status was tested with immunohistochemical staining method (IHC) in 214 pieces of tumor (92.24%), in which 62 was negative (28.97%). PR was tested with IHC in 213 pieces of tumor (91.81%) and 45 were negative (21.13%). In the outcome of detecting HER-2 status with IHC, “-”or “+” was defined as negative, “3+” as positive. In the FISH results of HER-2 with IHC, “2+” was not available in this study. The status of HER-2 was available in 197 pieces of tumors (84.91%) with 13 positive (6.60%). Fifty-six patients (24.14%) had axillary lymph nodes involved, in which 14 (25.0%) had 4 or more nodes positive. The remaining 176 patients (75.86%) had non-involved lymph nodes in axillary.
Treatments
All the patients received breast-conserving surgery (extensive lumpectomy or segmental mastectomy), and all received radiation therapy following the surgery except one. Nine patients with the primary tumor larger than 3 cm received neo-adjuvant chemotherapy, and then received the surgery when the tumor shrunk to 3 cm. Usually the tumors were resected along with 1-2 cm normal tissue around the tumor, and all the margins were negative conformed by the examination under microscope during the operation. Besides, the pectoral fascia under the tumor bed and the tract of puncture or incision for biopsy were resected during the operation. The spindle-shaped skin covering the tumor can be removed if necessary. When the tumor located in the upper part of the breast, an arc shaped incision parallel to the nipple-areolar would be selected, and when the tumors located in the lower part, a radiated incision selected. Another 5-6 cm long incision parallel to the axillary fold line was need when dissecting axillary lymph nodes. According to Berg standard, I and II levels of axillary lymph nodes were dissected.
Radiation treatment was arranged 3-4 weeks after the breast-conserving surgery, followed by chemotherapy. Some younger patients with 4 or more positive lymph nodes and lymphovascular invasion (LVI) etc. received chemotherapy right after the surgery. If the lymph nodes were negative, the patients only need the irradiation against the involved breast and the chest wall, and a total dose of 4,500-5,000 cGy was delivered during 4.5-5.5 weeks. After that an additional dose of 1,000-1,500 cGy was delivered to the shrunk field during 1-1.5 weeks. Additional dose to the supraclavicular area and the apex of axillary fossa should be delivered, if the lymph nodes were positive. Adjuvant multiagent chemotherapy was given to 159 patients (77.18%). The mainly used chemotherapy regimen was 4-6 cycles of cyclophosphamide, methotrexate and 5-fluorouracil (CMF) administered intravenously or cyclophosphamide, adriamycin and 5-fluorouracil (CAF) via the same way. Some patients received chemotherapy regimen of taxol and adriamycin (TA) delivered intravenously. Adjuvant hormonal therapy was advised to the patients who had positive hormonal receptors, and 141 patients (73.06%) in this study cohort received adjuvant tamoxifen (TAM) for 2 months to 5 years. Some patients received adjuvant aromatase inhibitors which replaced TAM while they reached menopause. TAM was stopped using less than 5 years in some patients because of intolerant side effects of the drug.
Follow-up
Most of the patients routinely visited the outpatient department of the hospital, and some patients were admitted to the hospital for chemotherapy or radiotherapy. These patients’ medical records were available and reviewed. Some information of the patients was obtained through telephone visit. The time of follow-up was counted from the day of receiving the breast-conserving surgery until the last unknown status. By Dec. 2008, the median time of follow-up was 54 months (range, 2-118 months).
End-points
The end-points assessed were local recurrence (LR), distant metastasis (DM), disease-free survival (DFS) and overall survival (OS). The LR was defined as ipsilateral breast tumor recurrence or ipsilateral regional nodal failure. A DM was defined as tumor metastasis to any other site other than ipsilateral breast or ipsilateral regional nodes. DFS was defined as survival without LR or DM. The DFS time began on the operation day and ended when LR or DM was diagnosed or the patient status was unknown. The OS time began on the operation day and ended when the patient died or the patient status became unknown. Contralateral primary breast cancer and other second primary tumor were not the DFS or OS end-point events.
Statistical analysis
Statistical analysis was performed using SPSS 10.0 software. The data were described with mean, median or percentage. Clinical characteristic analysis between the groups was performed using t-test, χ2 or Fisher exact test. These potential prognosis factors in the univariate analyses between the 2 groups consisted of tumor size (t-test), lymph node status (positive vs. negative), hormonal receptor status (positive vs. negative), HER-2 receptor status (positive vs. negative), AJCC phase (0, I and II phase vs. III phase), tumor pathological type (infiltrating versus non-infiltrating) and the use of neoadjuvant chemotherapy, adjuvant chemotherapy, radiation treatment and hormonal therapy (given versus not given). The survival status was analyzed with Kaplan-Meier method, and survival rates were compared between the groups using Log-rank tests. The multivariate analysis was performed with Cox regression model. The P value < 0.05 (two-tailed) was considered statistically significant.
Results
We compared the potential prognosis factors such as tumor size, lymph nodes status, hormonal receptor status, HER-2 receptor status, AJCC phase, pathological type and the use of neo-adjuvant chemotherapy, adjuvant chemotherapy, radiation treatment and hormonal therapy between the younger group and the elder group by univariate analysis, and the mean tumor size was compared with t-test (1.86 cm vs. 1.79 cm, t = 0.509, P = 0.518). There were no significant differences in the 2 groups. Details are shown in Table 1.
Clinical features of breast-conserving patients in younger group (≤ 35 years old) and elder group (> 35 years old).
By December 2008, the median follow-up time was 54 months. LR or DM presented in 15 patients of all the breast-conserving patients (15/232, 6.47%). In the younger group, LR or DM presented in 5 patients (5/37, 13.51%), including DM in 4 and LR in 1 companying DM. One patient in younger group received mastectomy after LR was diagnosed. In the elder group, LR or DM presented in 10 patients (10/195, 5.13%), including DM in 8 and LR in 2 with DM developed. As for the sites of metastasis, 3 cases had metastasis to the lung, 3 cases to the liver, 2 cases to the brain and 2 cases to the bone in the younger group; and 5 cases had metastasis to the lung, 2 cases to the liver, 3 cases to the brain and 6 cases to the bone in the elder group.
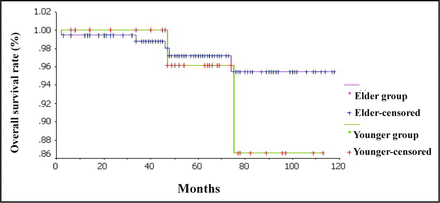
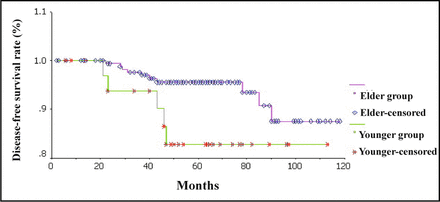
Two patients (5.41%) died in the younger group, and 5 (2.56%) in the elder group including 1 elder patient died for cardiovascular complications 2 months after the breast-conserving surgery. We analyzed the survival status of younger and elder patients with Kaplan-Meier method. The 5-year OS rate in the younger and elder groups was 96.08% and 97.19%, respectively, and there were no significant differences between the 2 groups (χ2 = 0.69, P = 0.4066). The 5-year DFS rate in the younger and elder groups was 82.58% and 95.52%, respectively, and the difference between the 2 groups was significant (χ2 = 4.02, P = 0.0451). The overall and disease-free survival curves are shown in Fig. 1 and 2, respectively. Since the DFS time in the younger group was shorter than that in the elder group, we analyzed the prognosis factors affecting the DFS of younger breast-conserving patients with COX regression model (Forward: LR method). We found that lymph node status was the only prognostic factor affecting the DFS of younger patients who received breast-conserving treatment (OR = 7.357, 95%CI: 1.030-52.563, P < 0.05). We also analyzed the prognosis factors affecting the DFS of all breast-conserving patients with COX regression model (Enter method). We found that lymph node status and the age of 35 years old or younger were the prognostic factors (OR = 3.467, 95%CI: 1.048-11.472, P < 0.05; OR = 0.245, 95%CI: 0.069-0.863, P < 0.05). Some details are shown in Table 2. Other prognosis factors including pathological type, tumor size, hormonal receptor status and HER-2 receptor status had no effects on the DFS of breast-conserving patients (P > 0.05).
Overall survival curves of younger group and elder group.
Disease-free survival curves of younger group and elder group.
Prognosis factors affecting the DFS of breast-conserving patients with Cox regression model.
Discussion
In China, breast-conserving surgery was originally used in the elder patients or patients in poor health condition which kept them from receiving major operation as the complications may develop. The treatment for the breast cancer patients has been developed with the further understanding of the disease. Halsted believed in his classical theory that breast cancer was a regional disease, of which tumor cells metastasized through lymph circulation, therefore, extensively and entirely removing the tumor and its lymph circulation was the essence in the surgery. Halsted’s theory was widely accepted until Fisher B introduced his theory. Fisher B’s study displayed that breast cancer was a systemic disease[2], and the tumor cells could metastasize to distant sites through blood circulation in the early period of the disease. The concept of minimal effective treatment was gradually accepted by oncologists and replaced the traditional concept of extensive resection. Without lowering the survival rate, the extent of the surgery was gradually shrunk. Based on the results of a series of randomly controlled study[1-3,6], breast-conserving therapy has become a standard treatment for the breast cancer patients in stage I and II world wide. Even some advanced breast cancer patients could receive breast-conserving surgery after the stage was lowered with neo-adjuvant chemotherapy[7-9]. In this cohort of the patients, 9 cases received neo-adjuvant chemotherapy followed by breast-conserving surgery. Analysis of our data displayed that the number of the cases receiving breast-conserving had been increasing since 1999, while the proportion of younger breast-conserving cases was small still.
Comparing the clinical features between the younger and elder breast-conserving patients, no significant difference was found in this study. These features consisted of tumor size, lymph node status, estrogen receptor, HER-2 receptor, AJCC stage, pathological type, neo-adjuvant chemotherapy, adjuvant chemotherapy, adjuvant radiotherapy and endocrinotherapy. The result was consistent with the findings of Han et al.[10] The choice of breast-conserving surgery for the patients in this study had not been affected by the age factor at the beginning, and the main factors which affected the choice of breast-conserving treatment were the tumor size, localization, lymph node status and the clinical phase. This may explain why there was no significant difference when these features were compared in the cohort of the patients. But the results of some other studies indicated that there were some differences between the two groups. Usually younger patients were inclined to having more advanced disease, more nodes involved and a higher positive proportion of HER-2[4,5,11], while some investigations displayed that the positive proportion of the hormonal receptor in younger breast cancer patients was higher than that in elder patients[12].
In our study, we found that the disease-free survival rates in the younger and in the elder breast-conserving patients were different, and local recurrence or distant metastasis presented more in the younger group than that in the elder group. We also found that positive status of lymph nodes and the age of 35 years old or younger were the prognosis factors affecting the DFS of breast-conserving patients. And in younger breast-conserving patients (≤ 35 years old), status of lymph nodes was the only prognostic factor affecting DFS. Many studies[13,14] also demonstrated that patients younger than 35-40 years at the time of diagnosis had a significantly higher risk of local recurrence after breast-conserving surgery than the elder patients. And El Saghir et al.[12] found that young patients (≤ 35 years old) with positive lymph nodes and positive hormonal receptors had a worse prognosis, whereas young age did not affect the survival of patients with negative lymph nodes or patients with negative hormonal receptors. Then which factors affect the prognosis of younger breast-conserving patients are uncertain, and enlargement of case amount and further follow-up should be required to achieve the final conclusion.
Thus, is it suitable for younger patients (≤ 35 years old) to receive breast-conserving surgery, while younger breast-conserving patients have had a poor DFS than the elder group? Beenken et al.[15] found that the type of surgery did not affect the locoregional disease-free or overall survival. Kroman et al.[16] found that the risk of local recurrence in younger breast-conserving patients was increased, but there was no increased risk of death caused by breast-conserving surgery in younger breast cancer patients when compared with that in the patients with radical mastectomy. In our study, the difference in the overall survival rates between the two groups was not found. When a little lower rate of DFS was acceptable, breast-conserving treatment could be a reasonable choice for younger patients. Is it acceptable? It is still debated.
In our study, we also found that many younger breast-conserving patients didn’t develop local recurrence or distant metastasis in the follow-up time. Are there some prognostic factors different among these younger patients? Nguyen PL’s study[17] found that ER or PR positive and human epidermal growth factor receptor 2 (HER-2) negative breast cancer patients (Luminal A subtype of breast cancer) had a lower rate of 5-year local recurrence (0.8%) than the patients with other subtypes of breast cancer, of which the rate of 5-year local recurrence was 1.8%. Zhou et al.[18] found that the higher rate of ipsilateral breast tumor recurrence in younger breast-conserving patients was due to more younger patients presented grade 3 when diagnosed. This suggests that traditional prognostic factors are not adequate for making the decision to conserve the breast, and the molecular subtype and the differential status of breast cancer should be considered. Usually, when the diagnosis of breast cancer is confirmed by fine needle biopsy and the patient is “suitable” for breast-conserving surgery, and then the operation will be done. The pathological information of the tumor carried to the surgeon team before operation is too little to judge the molecular subtype of the tumor, however, some cases with the subtype tumor which had more chance for local recurrence or distant metastasis received breast-conserving surgery. Maybe not only the malignancy but also the molecular subtype of breast cancer should be confirmed before the operation, and core needle biopsy is need before breast-conserving decision is made in younger patients.
Now surgeons should cautiously give the BCT to the patients especially those with positive lymph nodes, though younger breast cancer patients have more desire to conserve breast. And younger patients treated with breast-conserving surgery should receive aggressive post-surgical treatment when metastatic axillary lymph nodes, or negative ER, or peritumoral vascular invasion presents[19]. Even, systemic therapy should be considered for younger breast-conserving patients with negative axillary lymph node for reduction of LR[20]. Besides, the check-up in the hospital during and after the treatment for younger breast-conserving patients should be more frequent.
- Received May 4, 2009.
- Accepted July 31, 2009.
- Copyright © 2009 by Tianjin Medical University Cancer Institute & Hospital and Springer