Abstract
OBJECTIVE It is unclear whether differentiation disturbances or deregulation of neural stem cells (NSCs) are the early key steps for gllomagenesis and tumor development. Furthermore, relevant molecular changes and generegulation pathways are unknown. This study focused on screening and validating differentiation-associated genes from both human NSCs and glioma cells with malignant progression, for the purpose of offering an experimental basis for the cellular origin of gilomas and molecular pathology of gliomagenesis.
METHODS The differential-gene expression profiles of malignant progression of gliomas were established, then the differentiation related genes were screened out with a bioinformatics analysis. Expression levels of these genes was further analyzed In cultured human fetal NSCs undergoing differentiation processes with a semi-quantitative RT-PCR assay.
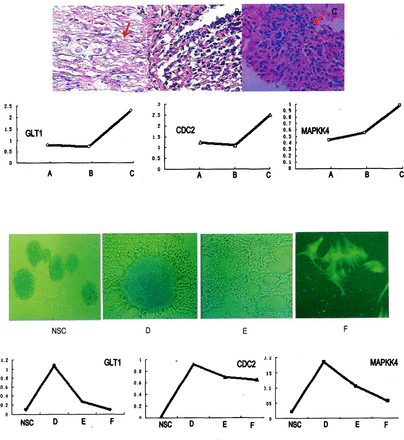
RESULTS Eight genes were screened out from the gene-expression profiling of which the expression levels were associated with the differentiation processes of NSCs, namely CXCR4, TN-C, GLT1, IL1-RI, EGFR-8, CDC2, Ndr3 and MAPKK4. Three of them, le., GLT1, CDC2 and MAP-KK4, were further analyzed, showing that expression levels decreased with the differentiation processes of NSCs, and increased with the malignant progression of ganglioglioma.
CONCLUSION Three differentiation associated genes were found negatively associated with NSCs differentiation and positively associated with malignant progression of gliomas, suggesting that differentiation disturbances of neural stem cells may be involved in oncogenesis, and that further studies on their roles in gliomagenesis should be conducted.
keywords
In the early 1990s, Reynolds and Weiss provided the initial evidence that multipotent neural stem cells of the adult rodent brain can be identified and isolated in vitro following mitogen stimulation. [1] There is no doubt that neural stem cells (NSCs) reside in the human developing or mature central nervous system. [2] Recently a small population of NSC-like tumor cells, termed brain tumor stem cells, were isolated from human brain tumor tissues, including glioblastoma, medulloblastoma and ependymoma.[3-6] Neural stem cells and brain tumor stem cells share the characteristics of continuous self-renewal, extensive brain parenchymal migration/infrltration, and the potential for frill or partial differentiation. The resemblance between NSCs and brain tumor stem cells provides new clues for studying the origin of gliomas.[7] If the common regulating genes responsible for cell proliferation and differentiation of these two kinds of stem cells were identified, it would be of great value for investigating the molecular pathology of gliomagenesis. In this report, we have examined common molecular changes during differentiation of NSCs and malignant progression of ganglioglioma, in order to identify target genes responsible for the etiology of gliomas.
MATERIALS AND METHODS
Clinical information
Following informed consent, tumors used in an RNA expression array were obtained from a 37-year-old female patient who had been undergoing surgical removal of a right temporal tumor. She underwent an operation three times from January 1999 to Februrary, 2001 in the Department of Neurosurgery, the 2nd Affiliated Hospital of Suzhou University. The subsequent pathological diagnoses were ganglioglioma, anaplastic astrocytoma and glioblastoma, based on paraffin embedded sections using World Health Organization 2000 (WHO) criteria. As approved by the Research Ethics Board at the 2nd Affiliated Hospital of Suzhou University, human fetal brain from a 7~10-week-old embryo was obtained from the consenting patient who received an induced abortion for medical reasons.
Gene expression profiling analysis
The gene-expression profiling of malignant progression from ganglioglioma to glioblastoma was established following our previously reported methods,[8] which contained 16,363 cDNA clones. Of these, there were 489 differentially expressed genes showing a differential ratio value greater than 3.0. These clones were screened out in the malignant progression profiling, then the developmental and differentiation associated genes were analyzed through cluster analysis of bioinformatics.[9, 10] The candidate genes involved in malignant progression of ganglioglioma through dysrégulation of cellular differentiation and developmental disorder were further selected out by a bioinformatics assay.
Magnetic cell sorting and flow cytometry
The cell suspension was passed through a 30 μ m nylon mesh, washed once and resuspended in PBS containing 2 mM EDTA. Using a Miltenyi Biotec CD133 Cell Isolation kit, magnetic labeling was conducted with 1μ CD133 MicroBeads/106 cells. Magnetic separation was carried out on LS columns (Miltenyi Biotec). Positive fractions were collected, and aliquots of CD133+ sorted cells were evaluated for purity by flow cytometry with a FACSCalibur instrument (BD Biosciences).
Expression of developmental and differentiation-associated genes during NSCs differentiation
Based on methods we previously reported, [11, 12] a CD133+ single cell suspension, derived from the brain tissues of a 7~10-week-old aborted human embryo was cultured in N2-DMEM-F12 medium supplemented with EGF and bFGF. The neurospheres formed in the cell culture were transferred, then divided into two groups and cultured further in N2-DMEM-F12 culture media supplemented with either EGF or 10% fetal bovine serum. Different cell groups associated with the differentiation process were identified by detecting the relevant specific cell surface marker with an immunohistochemical assay, i.e., CD133 and Nestin for NSCs, NSE for neurons and GFAP for astrocytes. The neural cells, cultured in vitro from different differentiation stages and status, were collected to analyze the expression of developmental and differentiation associated genes with a semi-quantitative RT-PCR assay, according to the methods we reported previously.[13] Quantification of GAPDH transcripts as an internal control for the amount and quality of cDNA was performed for all samples. Detailed information concerning primer sequence and product size is available upon request. The primer sets were all optimized to generate only a single specific band on argarose gel from the cDNA. Melting-curve analysis was performed in all the reactions for identifying the specificity of amplifications. All experiments were performed in duplicate.
Results
Screening of the target genes
After cluster analysis of the gene-profiling data, eight genes associated with cell ontology and differentiation were screened and are indicated in Table 1. The expression status of these genes at the different differentiation stages of NSCs were analyzed with a semiquantitative RT-PCR assay (Table 2).
Gene chip data of differentially expressed genes associated with development and differentiation during tumor progression into different glioma malignancy grades
Expression changes of development and differentiation associated genes in the differentiation process from NSCs to astrocytes with a semi-quantitative RT-PCR assay
Expression tendency analysis of genes associated both with differentiation of NSCs and malignant progression of ganglioglioma
CD133+ cells were seeded in serum-free medium supplemented with EGF and bFGF. Then the cells gradually formed into suspended cell spheres. Most of the cells in the cell spheres were NesthT and GFAP’, with only a small fraction being CD133+. In the adherent cell spheres, a small population of GFAP+ cells appeared 7 days later when cultured in vitro supplemented only with EGF, while a majority of the cells were still Nesting Then the culture medium was supplemented with 10% fetal bovine serum instead of EGF and the cells were continuously cultured in vitro for another 7 days. Under these conditions, the GFAP+ cells reached approximately 91.36 ±1.55%, while the NesthT cells decreased to only 4.64 ±0.60%. With 14 more days of culture, GFAP+ cells rose to 98.31 ± 1.80%, and the NesthT cells and NSE+ cells could not be detected. Different populations of cultured cells experiencing various differentiation stages can be identified according to the results of immunohistochemical staining, namely, undifferentiated NSCs were CD133+, NSCs in an early differentiation stage were CD133+and NesthT, glial progenitor cells were weakly positive in both GFAP and Nestin staining, while only astroglial cells were GFAP+. Expression of genes associated with malignant progression of gliomas were analyzed in these different differentiated cell populations with a semi-quantitative RT-PCR assay. Three genes closely associated with both differentiation of NSCs and malignant progression of gliomas were screened out, namely, GLT1, CDC2 and MAPKK4. The expression level of these three genes increased along with the malignant progression of gliomas, and decreased with the differentiation processes of neural stem cells. The tendency for expression of these three genes in tumor specimens during malignant progression in different malignancy grades, and in vitro cultured cells with different differentiation stages were further analyzed according to the data obtained from the gene chip, RT-PCR, pathological examinations and immunofluorescence assay (Fig. 1).
Correlations of NSCs differentiation and malignant progression of ganglioglioma.
Upper half part: Above: pathological examination of glioma specimens In different malignancy grades during tumor progression (A-C) and expression of 3 genes in these corresponding specimens. A primary tumor (H&E staining, 400 * ): neoplastic neurons, neoplastic astrocytes and binucleate neurons (T) can be seen. Pathological diagnosis was ganglioglioma (WHO grade II). B 1st recurrent tumor(H&E staining, 400 * ): Neoplastic neurons disappeared, while neoplastic glial cells Increased with high density. Nuclear heteromorphism can be seen and chromatin obviously increased. Pathological diagnosis was anaplastic astrocytoma (WHO grade III). C 2nd recurrent tumor (H&E staining, 400x ): Microscopic field was crowded with neoplastic astrocytes, forming into vascular retia of chicken wire permutation, and pseudopalisade formation and multlnucleated giant cells (t) were found. Pathological diagnosis was glioblastoma multiform (WHO grade IV). Below: gene expression ratio values of GLT1, CDC2 and MAPKK4 in gene expression profiling corresponding to the tumor specimens above. Gene expression values rose with malignant progression of glioma.
Lower half part: Above: undifferentiated neural stem cell sphere (NSC), neural stem cells initiating differentiation (* 200), the majority were Nestin’ cells, while the minority were GFAP* cells(D); neuroglial progenitor cells (* 200), GFAP’ cells were predominant, with few NSE* cells and Nestin’ cells (E); terminal differentiated mature astrocytes (GFAP immunofluorescence staining, * 1000) NSE* cells and Nestin’cells could not be found. Below: Expression of three genes, GLT1, CDC2 and MAPKK4 In cells with different differentiation stages, corresponding to the four cell populations mentioned above. Expression levels of these three genes was very low In undifferentiated NSCs sphere (NSC), but Increased In NSCs Initiating differentiation(D), then decreased gradually in neuroglial progenitor cells (E) and astrocytes (F).
Discussion
Numerous studies have focused on the development and differentiation of cells of the central nervous system. Many studies have shown that a special type of stem cells can transdifferentiate into another type of cell under certain conditions, such as bone marrow derived stem cells which can migrate into the brain parenchyma, and transdifferentiate into mature astrocytes expressing GFAP, when they were injected into the lateral ventricle of neonatal mice.[14] It has been shown that human embryonic NSCs can transdifferentiate into skeletal muscular cells when cocultured with sarcoblasts in vitro.[15] NSCs can also transdifferentiate into a variety of hematopoietic cells, including the myeloid and lymphoid cell lineages, as well as more immature blood cells and human and rodent bone marrow stromal cells can transdifferentiate into cells bearing neuronal markers.
When the GFAP+ cells, derived from differentiation of NSCs in vitro, were transplanted into the brain, they maintained the features of astrocytes if they localized in the gray matter and turned to oligodendrocytes if they resided in the white matter.[16] The GFAP+ cells can exhibit the features of neuron-like cells, if they localized in the subependymal region, sometimes even in the brain cortex.[17] To some extent, these results suggested instability of the differentiated state in CNS cells. In some conditions differentiated cells may not remain in their expected stable differentiated stage, i.e. de-differentiate and/or transdifferentiate into another type of differentiated cell. The mechanism of this interesting phenomenon is of great interest to neurophysiologists and neurooncologists.
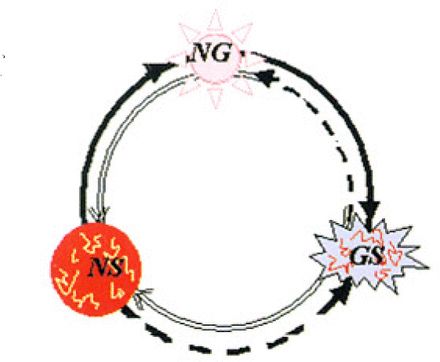
Our interests have related to this problem, so we have investigated the relationship among NSCs (NS), glioma cells (GS) and normal glial cells (NG) in glioma patients and raised the hypothesis that the three kinds of cells can transform into any of the other two kinds of cells under certain circumstances as noted in Fig.2.[18].
Relationships among NSCs (NS), glioma cells (GS) and normal glial cells (NG).
The transformation directions (solid arrowhead) have been generally accepted up to now and directions showed by dashed arrowhead are now under confirmation. The directions with double-line arrowhead need to be confirmed in the future. Especially, the possibility of the transformation between GS and NS cells has been proposed and research on the relevant molecular mechanisms is on the way.
From the studies on neural cell differentiation, we can predict the possibility that the transformation could take place along the directions showed in Fig.2, either clockwise or counter clockwise among those three kinds of cells. At least, the molecular basis of some transformations exist. Some transformations can not take place because of insufficient transformation conditions. For example, there is no doubt about the conversion of NS into NG cells, while the conversion of NG into NS cells was thought impossible in the past, but that at least parts of the steps in the differentiation process from NS to NG cells were reversible.[17]Now there is still no direct proof of the conversion of GS into NS cells, but Singh et al.[3, 4] found that stem cells existed in gliomas, and to some extent, had a common molecular phenotype (such as Nestin and CD133) with NSCs. These tumor stem cells had more biological characteristics similar to the original tumors.[19]
Our studies first showed that three genes, CDC2, MAPKK4 and GLT1, are involved in both the differentiation of NSCs and the dedifferentiation of ganglioglioma. The expression level of these genes decreased gradually with differentiation from NSCs towards terminal mature glial cells, and increased along with the dedifferentiation process from ganglioglioma towards glioblastoma. Although the tendency of expression of these genes in the two processes were different, they in common shared that they were all genes with a high level of expression, both in undifferentiated glial and glioma cells. So we can deduce that the more common features these stem cells (tumor and normal stem cells) shared at a molecular level, more possibilities there were for sharing the same origin and could transform between each other.[20] Particularly, the three genes, CDC2, MAPKK4 and GLT1, which were closely associated with development and differentiation and were screened out from the gene expression profiles associated with malignant progression of ganglioglioma,[21] were also highly correlated with NSCs differentiation. Moreover, the initiating cells of both neoplastic neurons and neoplastic astrocytes existing in gangliogliomas, were likely to be their common precusor cells—NSCs. These data provided not only the molecular evidence for the conversion from NS to GS cells, but also was valuable for studying the possibility of conversion from GS to NS cells or GS to NG cells.
It is well known, that there are two patterns of proliferation and differentiation of NSCs: one is a symmetric division pattem, the other is an asymmetric division pattem. Asymmetric division leads to cell differentiation.[22] If tumor stem cells also follow this mle, it would be hopeful for tumor prevention and treatment. Accordingly, many intervention therapies could be utilized to induce tumor stem cells to follow an asymmetric division pattem as much as possible or completely inhibit symmetric dividing of tumor stem cells.
Footnotes
This work was supported by the National Natural Science Foundation of China (No. 30371457, 30400457).
- Received November 16, 2006.
- Accepted December 15, 2006.
- Copyright © 2006 by Tianjin Medical University Cancer Institute & Hospital and Springer