Abstract
Esophageal cancer (EC) is an aggressive malignancy with a poor prognosis. Various factors, including dietary habits, and antacid and antibiotic use, have been shown to influence the esophageal microbiome. Conversely, enrichment and diversity of the esophageal microbiome can also impact its function. Recent studies have revealed prevalent changes in the esophageal microbiome among patients with EC, thus suggesting the potential contribution of the esophageal microbiome to EC development. Additionally, distinct microbiome compositions have been observed in patients with different responses to radiotherapy and chemotherapy, indicating the role of the esophageal microbiome in modulating treatment outcomes. In this review, we have examined previous studies on the esophageal microbiome in healthy individuals and patients with EC or other esophageal diseases, with a focus on identifying microbial communities associated with EC pathogenesis and prognosis. Understanding the role of the microbiome in EC may aid in early detection and optimized treatment strategies, ultimately leading to better outcomes for patients.
keywords
Introduction
Esophageal cancer (EC) is the seventh most common cancer and the sixth leading cause of cancer-related deaths worldwide1. Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) are the two primary subtypes of EC, but ESCC and EAC differ significantly with respect to geographic patterns, temporal trends, and risk factors2,3. ESCC is prevalent in central Asia and the easterly-lying corridor in Africa, extending from the Taihang Mountains in northern China to central Asia, Iran, and eastern and southern Africa2. In contrast, EAC is more prevalent in industrialized countries in Europe3.
The symptoms of EC are correlated with disease progression. In the early stages, patients may be asymptomatic or complain of dysphagia, while in the advanced stages patients may exhibit progressive dysphagia, persistent retrosternal or back pain, and marked cachexia. Endoscopic therapy is routinely used to remove cancerous tissues in early-stage disease. Chemotherapy and radiation therapy are usually applied before or after surgical resection for locally advanced disease4, while treatment options for metastatic disease include chemotherapy, radiation therapy, targeted therapy, immunotherapy, or a combination of these treatments to manage symptoms and slow disease progression. The use of neoadjuvant radiotherapy and chemotherapy during the perioperative period can effectively prolong the median survival rate for patients with advanced disease, particularly patients with ESCC. Surgical resection after neoadjuvant radiotherapy and chemotherapy has become the standard treatment for locally advanced ESCC5.
Several factors are associated with an increased risk of EC (Table 1). The incidence of ESCC is typically higher in men, while women may be more susceptible to developing EAC; however, the exact mechanisms underlying this difference are unclear6. Smokers have a five-fold higher risk of EC than non-smokers7. Alcohol consumption does not appear to be a significant risk factor for both ESCC and EAC2. Obesity, particularly central obesity, may increase intra-abdominal and gastric pressure, promote gastroesophageal reflux, and promote the development of EAC10. Individuals residing in developing countries or regions with limited access to fruits and vegetables have increased susceptibility to esophageal inflammation and cancer2. Individuals with low levels of education, physical activity, and income have a higher risk of EAC in developed countries; currently, a similar trend has been noted in developing countries9,11. More recently, researchers have studied alterations in esophageal microbiota that may contribute to the development of EC.
Known risk factors for esophageal carcinoma
Culture-independent high-throughput DNA sequencing technologies, coupled with advanced computational tools, empower the comprehensive analysis of the intricate human microbiome11. It is well-established that the gut microbiome, which consists of trillions of microorganisms, exerts a significant influence on facilitating the development of gastrointestinal tumors and suppressing antitumor immune responses12; however, the potential contribution of esophageal microorganisms to the development of EC has yet to be fully elucidated. One of the hurdles in studying the esophageal microbiome involves obtaining samples from the esophageal epithelium because this procedure is invasive and may result in complications13. Herein we provide a comprehensive overview of our current understanding of the microflora present in healthy and malignant esophageal tissues, along with insight into other prevalent esophagus-related disorders.
Factors influencing the esophageal microbiome
The esophagus serves as a crucial conduit linking the oral cavity to the stomach, and the esophageal microenvironment undergoes dynamic fluctuations14. The composition of microbial communities within the esophagus can be influenced by various factors, such as dietary habits. Urban populations, which are characterized by a high consumption of fatty and processed foods, have elevated levels of Bacteroides, along with diminished levels of Firmicutes. Rural populations adhering to a well-balanced diet rich in fiber have increased levels of Prevotella, Treponema, and Succinobacterium, which aid in the breakdown of polysaccharides and dietary fiber. Prolonged adherence to an urban-style diet can lead to chronic esophageal inflammation and dysbiosis, potentially contributing to the progression of esophageal diseases15.
Gastroesophageal reflux disease (GERD), a significant risk factor for Barrett’s esophagus (BE), can reshape the microecology of the cardia and esophagus due to the reflux content, which is primarily composed of gastric acid and thus creates an acidic environment14. The impact of medications on the esophageal microbiota should not be underestimated. Proton-pump inhibitors (PPIs) have been shown to cause a notable increase in Streptococcus and a decrease in Gram-negative bacteria after use, resulting in reduced inflammation and ulceration in the lower esophagus16. The combination of omeprazole and antibiotics significantly reduces flora in the lower esophagus of mouse models, leading to the absence of specific bacterial colonization17. This finding is likely due to the antibiotics targeting Helicobacter pylori and other bacteria, or altering the microenvironment in a manner unfavorable for some bacterial populations. Other factors that influence the microbial compositions in the esophagus include obesity, autoimmune disorders, and surgical interventions18.
Bacterial community of a healthy esophagus
The esophagus, a muscular tube connecting the pharynx and the stomach, serves as a conduit for the transport of food and liquids. The esophagus can be divided into distinct upper, middle, and lower segments. The inner lining of the esophagus is comprised of a stratified squamous epithelium. Investigating the microbial composition in the esophagus and unraveling the biological functions poses challenges due to its unique anatomy. Traditional culture-based studies have suggested that the esophagus lacks permanent microbial inhabitants, with only a limited presence of transient bacteria acquired through swallowing or gastroesophageal reflux19.
In 2004, Pei et al.20 identified 95 bacterial species from 6 phyla (Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and TM7) in the distal esophagus using 16S rDNA sequencing (Figure 1). In 2009, Yang et al.21 investigated the distal esophageal microbiota in 12 healthy individuals and classified the microbiota into 2 subtypes. Type I microbiota is primarily dominated by Gram-positive bacteria, particularly Streptococcus, which is typically distributed in the esophagus21. Type II microbiome is mainly Gram-negative bacteria presenting in diseased esophagus. Streptococcus, along with other genera, such as Prevotella and Lactobacillus, appears to be one of the dominant taxons in the normal esophageal microbiota, while Peptostreptococcus, Neisseria, and Actinobacillus are less abundant and rarely reported22. In 2013, Norder et al.23 compared the flora of the lower esophagus, the upper esophagus, and the oral mucosa in healthy individuals, and reported that the composition of the microbiota in these locations was almost comparable. According to a review by Wang et al.,24 other studies have shown that the esophageal microbiota could be affected by adjacent compartments. It is estimated that approximately one bacterial cell per day flows from the mouth to the stomach25, and the microbial composition overlaps between the mouth, pharynx, esophagus, and gut14,23. Specifically, Streptococcus, Neisseria, Prevotella, Actinomyces, and Welchella are the most abundant species in the oral cavity and esophagus26. The studies that have investigated the microbiota in a healthy esophagus are summarized in Table 2.
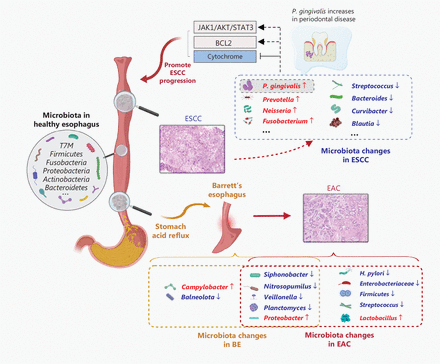
Microbiota changes in esophageal cancer. The microbiota of the distal esophagus was influenced by acid reflux from the stomach. Acid reflux leads to inflammation and mucosal damage, resulting in the change of microbiome in the distal esophagus. This process allows the columnar epithelium to replace the original squamous epithelium of the esophagus, which can then progress to BE and EAC. The microbiota in the upper part of the esophagus is influenced by oral resident flora, in which P. gingivalis promotes the development of ESCC. BE, Barrett’s esophagus; EAC, esophageal adenocarcinoma; EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma.
Studies on the esophageal microbiota in healthy individuals
Esophageal microbial alterations in BE and EAC
Acid reflux-induced inflammation and mucosal damage can lead to GERD and subsequent replacement of the original esophageal squamous epithelium with columnar epithelium that has the potential to progress to BE27 (Figure 1). Several studies have identified Campylobacter enrichment in BE patients, but not in healthy individuals16,21,28. Conversely, Siphonobacter, Balneola, Nitrosopumilus, and Planctomyces have been shown to more abundant in healthy individuals than BE patients29. Yang et al.21 demonstrated that BE is primarily associated with an increase in Gram-negative anaerobic bacteria within the esophagus. The presence of lipopolysaccharide (LPS) on the surface of Gram-negative bacteria activates the NF-κB pathway, leading to elevated expression of IL-8, which is a significant event in the transition from a normal esophagus to BE. Taken together, these studies suggest that BE-induced microbiome changes in the esophagus may contribute to the development of EAC.
Different studies regarding this hypothesis have generated inconsistent conclusions. A study that involved BE and EAC patients in different stages of disease did not demonstrate a significant difference in the alpha diversities13. Snider et al.17 reported a notable decrease in alpha diversity in high-grade intraepithelial neoplasia and adenocarcinoma compared to non-cancerous esophageal tissue. In addition, a decrease in Firmicutes and an increase in Proteobacteri were also demonstrated during the progression from BE to EAC17 (Figure 1). Snider et al.17 also examined changes in the oral microbiota of BE patients, uncovering distinct taxonomic differences, such as an increased abundance of Streptococcus, Veillonella, and Enterobacteriaceae, and decreased Neisseria, Lautropia, and Corynebacterium. Notably, the combination of Lautropia, Streptococcus, and Bacteroides exhibit a high accuracy in identifying BE patients, with a sensitivity of 96.9% and a specificity of 88.2%30. Therefore, alterations in microbiota may serve as a useful tool for diagnosing and monitoring disease progression in patients with BE.
Microbial alterations in EAC
Microbial differences between BE and EAC
BE significantly increases the risk of developing EAC up to 30-fold compared to individuals without BE31. Numerous studies have demonstrated notable differences in the microbial composition between healthy individuals, BE patients, and patients with EAC. A comparison of the esophageal microbiota between EAC and BE patients showed reduced diversity in EAC patients, with decreased abundances of Veillonella and Streptococcus granulosa, while Lactobacillus emerged as the dominant flora influencing the local microenvironment13 (Figure 1). Of note, the researchers did not detect a significant difference between samples collected from fresh frozen tissues/endoscopic brushings and samples collected using a cyto-sponge, indicating that the cyto-sponge may serve as an alternative method for collecting esophageal microbiota13.
Lactobacillus is typically considered to be a part of the resident flora in the stomach. BE originates from the gastric cardia and chronic inflammation in this region exposes Lactobacillus to a more acidic environment32. Adaptation of lactic acid bacteria to this low pH environment allows the bacteria to thrive, leading to proliferation and the production of lactic acid through carbohydrate fermentation. This process further acidifies the environment, thus inhibiting the growth of other microorganisms and establishing lactic acid bacteria as the dominant flora33. The relative abundance of Gram-negative bacteria and Enterobacteria increases simultaneously, progressively intensifying as the disease worsens17,29. Conversely, several bacterial species, such as Siphonobacter, Algae, Nitrobacter, and Planckia, are significantly reduced in patients with EAC29 (Figure 1). These findings support the previous study by Yang et al.21, which revealed a higher proportion of type II microorganisms in diseased esophagus compared to type I microorganisms. These studies are summarized in Table 3.
Studies involving the esophageal microbiota in different esophageal diseases
Role of H. pylori in EAC development
H. pylori has been established as a carcinogen that is closely associated with the progression of various gastric disorders, including gastritis, gastric ulcers, atrophy, and adenocarcinoma37. Although H. pylori primarily colonizes the gastric mucosa, the presence of H. pylori can influence the microbial composition of the lower esophagus. Tian et al.38 demonstrated that H. pylori does not replicate in the esophagus, but has the ability to influence the diversity of the esophageal microbiota. Furthermore, several studies have reported a lower incidence of EAC in individuals infected with H. pylori compared to individuals who are uninfected39–41 (Figure 1). This epidemiologic evidence suggests that H. pylori infection might be a protective factor against the development of EAC.
Although the precise mechanisms underlying this inverse correlation between H. pylori infection and EAC remain unclear, researchers have proposed various explanations for this phenomenon. First, H. pylori may counteract the effect of factors that contribute to chronic inflammation and cancer, such as cytotoxin-associated gene A, vacuolated cytotoxin (VAC), and adhesins37. In addition, H. pylori has been observed to stimulate cancer cell apoptosis through Fas caspase, thus offering host protection42. Third, the presence of H. pylori has been linked to serum ghrelin levels. Eradicating H. pylori can lead to an increase in serum ghrelin levels, potentially contributing to obesity and affecting gastric emptying, thereby elevating the risk of BE and EAC41. Furthermore, some studies have linked this inverse correlation with H. pylori-induced atrophic gastritis, which can reduce gastric acid secretion and lower the risk of EAC, although this viewpoint remains controversial43.
Microbial alterations in ESCC
Differences between tumor and non-tumor tissues
Shao et al.34 reported reduced microbial diversity in ESCC tissues compared to non-tumor tissues, as determined by 16S rDNA sequencing, with a significant increase in the abundance of Fusobacterium and a decrease in Streptococcus abundance (Figure 1). In 2020, Li et al35. collected paired saliva and brush specimens from 82 healthy individuals, 60 patients with low-grade dysplasia (LGD), 64 patients with high-grade dysplasia (HGD), and 70 ESCC patients, to examine the microbiota at different stages of ESCC. Li et al35. revealed significantly decreased abundance of Streptococcus and increased abundance of Neisseria and Porphyromonas during the progression of ESCC. Specifically, Streptococcus and Neisseria could better predict the development of disease than other genera with reasonable specificity and sensitivity35. Another study conducted by Jiang et al.36 in 2021 that included 68 individuals (controls, n = 21; esophagitis, n = 15; and ESCC, n = 32) who underwent esophagectomy reported contrasting results. Jiang et al.36 reported an increase in Streptococcus spp. but a decrease in Faecalibacterium, Bacteroides, Curvibacter, and Blautia in ESCC tissues (Figure 1). These discrepancies could be attributed to differences in dietary habits, geographic locations, and variations in the number of patients included in the respective studies. Nevertheless, all these studies collectively demonstrated that alterations in the microbial equilibrium within the esophagus are prevalent in patients with ESCC.
Subsequent studies have indicated that a diminished microbial population serves as a microbial dystrophy index, enhancing the differentiation between EC and a healthy esophagus44. Functional analysis of the microbial composition in ESCC has revealed a decline in the activity of nitrate and nitrite reductases, which are closely linked to carcinogenesis through the production of reactive nitrates and nitrites45. Although these findings imply that microbial dysbiosis may contribute to the development of ESCC, further research is warranted to ascertain the specific microbiota involved and elucidate the underlying mechanisms.
Influence of Porphyromonas gingivalis in ESCC development
In 2016, Gao et al.46 investigated the presence of antigens, DNA, and periodontal pathogens in ESCC lesions and showed that the presence of P. gingivalis is more prevalent in ESCC than para-cancerous tissues (61% vs. 12%) but absent in the esophageal epithelium46 (Figure 1). Furthermore, the abundance of P. gingivalis varies across different stages of ESCC, with higher levels observed in patients with poor differentiation, severe lymph node metastasis, advanced stage disease, and a short survival cycle. These findings suggested that P. gingivalis could serve as a novel prognostic indicator for ESCC. Peters et al.47 determined the oral bacteria present in 25 pairs of patients with ESCC and healthy controls. The study revealed a higher prevalence of P. gingivalis in ESCC tumor tissues compared to paired healthy controls47. In addition, elevated levels of IgG and IgA antibodies against P. gingivalis were detected in ESCC patients compared to healthy controls. Notably, patients with high antibody levels exhibited a more favorable prognosis than patients with low antibody levels. These findings suggested that P. gingivalis may have a role in the pathogenesis and progression of ESCC48.
A recent study performed by Chen et al.49 determined the presence of P. gingivalis in the esophagus of 156 ESCC patients using immunohistochemistry to investigate the possible association between P. gingivalis infection and patient clinicopathologic features. Chen et al.49 detected P. gingivalis in 57% of the ESCC patients, and the infection contributed to EC development by promoting IL-6 production to induce the epithelial-mesenchymal transition and attract myeloid-derived suppressor cells. It is worth noting that P. gingivalis interacts with EC epithelial cells in different ways. For example, P. gingivalis secretes nucleoside diphosphate kinase to promote carcinogenesis50 and inhibits epithelial cell apoptosis through different pathways, such as activation of Jak1/Akt/Stat3 signaling51, enhancement of Bcl-2, and blocking the release of cytochrome c52 (Figure 1). Therefore, it is reasonable to consider P. gingivalis as a promising target to prevent and/or treat P. gingivalis-infected patients with ESCC49.
Microbiota in association with the prognosis of ESCC
Liu et al.53 explored the presence of esophageal microbiota in ESCC patients at different pathologic stages in an attempt to identify potential microbial markers with prognostic value. The findings revealed significant differences in the abundance of bacterial phyla and genera between patients with lymph node metastasis (N+) and patients without lymph node metastasis (N−). Notably, Bacteroidetes, Pleurotus, and Spirochetes had a higher abundance in N+ patients, while Proteobacteria exhibited a lower abundance in N+ patients compared to N− patients (Figure 1). At the genus level, Prevotella and Treponema were more abundant in the N+ group, while Streptococcus exhibited a higher abundance in patients with T3-4 tumors compared to T1-2 tumors. No significant differences were observed in the abundance of other genera. Additionally, the analysis indicated that the combined abundance of Streptococcus and Prevotella was associated with poor survival, suggesting that these genera could potentially serve as independent prognostic indicators for ESCC53.
Periodontal disease has been recognized as a significant risk factor for EC. Recently, increasing attention has been given to the potential involvement of oral flora in EC development. Notably, Fusobacterium nucleatum, a pathogen commonly associated with periodontal disease and colorectal cancer54, has been extensively studied (Figure 1). Yamamura et al.55 detected F. nucleatum DNA using PCR in 23% (74/325) of ESCC resected specimens, primarily from advanced-stage cases. The presence of F. nucleatum appeared to be independent of other factors, such as gender, age, cigarette smoking, alcohol consumption, and preoperative treatment56. Moreover, higher levels of F. nucleatum were observed more often in relapsed cases, and these patients had a lower survival rate compared to F. nucleatum-negative individuals55. Subsequent investigations focusing on ESCC patients after radiotherapy and chemotherapy revealed that those with a high abundance of F. nucleatum experienced more adverse reactions to chemotherapy, had a higher recurrence rate, and a shorter survival time compared to patients with a low F. nucleatum abundance, suggesting the potential contribution to chemoresistance57. These findings collectively support the notion that F. nucleatum holds promise as a prognostic factor for ESCC.
Effect of human papillomavirus (HPV) infection on ESCC
HPV infection has been closely linked to oropharyngeal squamous cell carcinoma58. Considering the similarity in human leukocyte antigen expression between squamous epithelial cells in the oropharynx and esophagus, it is reasonable to propose that HPV can potentially contribute to the development of ESCC. A case-control study conducted in Shaanxi, China aimed to detect HPV antigens in the blood and revealed significantly higher serum reactivity in ESCC patients compared to healthy controls59; however, subsequent studies on this topic have yielded conflicting results. Specifically, a study by Kamangar et al.39 in 2006 detected HPV antibodies in < 15% of serum samples from Chinese ESCC patients, and no definitive correlation between HPV and ESCC was established. Similarly, a study by Halec et al.60 in 2016 investigating the potential association between HPV and ESCC found no significant association. These studies used different methods to detect HPV infection in ESCC tissues and consistently observed low viral loads in cancer tissues. Furthermore, the presence of HPV DNA, mRNA, and subsequent p16 upregulation was not consistently observed. Based on the collective findings, the association between HPV infection and the incidence of ESCC appears to be weak.
In contrast, Sitas et al.61 utilized a centralized multiple serology method to analyze sera from 1561 patients with ESCC and 2502 controls. Sitas et al.61 found that ESCC was only associated with E6 for HPV16 and HPV6, but not with other types of HPV. Similarly, a study by Zhang et al.62 concluded that only individuals who were cigarette smokers, consumed alcohol, and were infected with HPV had a higher likelihood of developing ESCC. This finding suggested that HPV infection alone may not be an independent risk factor for ESCC but could potentially have a synergistic effect with other factors. The current epidemiologic and etiologic evidence supporting the association between HPV infection and ESCC remains inconclusive, and the topic continues to be a subject of discussion.
Dysbiosis facilitates the development of EC by disturbing the immune response
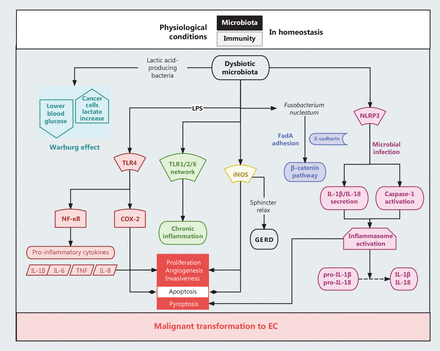
Changes in the composition and abundance of esophageal microbiota could promote the development of EC in different ways. For example, several lactic acid-producing bacteria, such as Staphylococcus and Lactobacillus, have an increased abundance in EAC tissues, which converts the high load of glucose taken up by cancer cells into lactate, thereby supporting the survival and proliferation of malignant cells63 (Figure 2). While extensive research is currently focused on investigating the precise mechanisms by which esophageal microbiota contribute to the development of EC, a prevailing observation is that the presence of chronic inflammation and compromised immune responses establishes a conducive microenvironment for this malignant transformation. The involvement of disrupted cross-talk between esophageal microbiota and immune cells has been implicated in several signaling pathways known to contribute to the development of EC.
A diagram on the interactions between esophageal microbiota and immune cells to promote the development of EC. Changes in the composition and abundance of esophageal microbiota promote the development of EC in different ways. Notably, increased lactic acid-producing bacteria, such as Staphylococcus and Lactobacillus in EC tissues, support tumor survival and proliferation by converting glucose into lactate. Chronic inflammation and compromised immune responses in the esophageal microenvironment create a conducive setting for malignant transformation. Dysregulation of the Wnt/β-catenin pathway by Fusobacterium nucleatum contributes to disease progression and treatment resistance through elevated production of chemokines. The altered esophageal microbiota activates multiple TLRs (TLR1, 2, and 6) and NLRP3 (a component of inflammasomes) to disturb the local microenvironment homeostasis and promote malignant cellular behavior.
Aberrant activation of the Wnt/β-catenin pathway has been implicated in the carcinogenesis and therapeutic resistance of EC64. Fusobacterium nucleatum, a bacterium capable of activating the β-catenin pathway through the production of FadA adhesion and modulating the inflammatory response, has been reported to be present in approximately 23% of patients with EC55. The tumor tissues of these affected patients have increased production of chemokines, which contributes to a more aggressive disease course and reduced survival55. In addition, changes in esophageal microbiota have been shown to facilitate the activation of multiple Toll-like receptors (TLRs) through different approaches. TLR4 activation led to increased NF-κB activities and cyclooxygenase-2 (COX-2) expression. The former promotes secretion of several chemokines, such as IL-6, IL-8, and TNF, while the latter is known to be associated with different malignant cellular phenotypes, including increased proliferation, angiogenesis, invasiveness, and decreased apoptosis65. TLR1, 2, and 6 induce inflammatory responses, the upregulation of which as been reported in EAC tissues. Of note, the TLR1, 2, and 6 network has also been implicated in identifying dysbiotic microbial components66. Third, Gram-negative bacteria stimulate inducible nitric oxide synthase (iNOS), the expression of which has been shown to be increased in EAC tissues compared to normal esophagus67. Depletion of Nod-like receptor protein 3 (NLRP3), a component of inflammasomes, causes aberrant bactericidal activities. Conversely, NLRP3 activation allows Tregs to maintain homeostasis by enhancing the secretion of IL-1β to neutralize the inflammatory response68 (Figure 2). Taken together, these studies indicate a close relationship between esophageal dysbiosis and aberrant immune responses, ultimately leading to the malignant transformation of EC, as reviewed by Sharma et al.69
Summary and outlook
The field of esophageal microbiology remains relatively unexplored, and the causal relationship between EC and the microbiome remains uncertain. Nevertheless, the studies reviewed in this paper have provided insight into the microbial composition of the normal esophagus and identified changes in microbial composition among EC patients, which is largely characterized by a reduction in bacterial species. There is no doubt that emerging advances in technology and innovative tools are progressively enhancing our understanding of the intricate involvement of the microbiota in the pathogenesis of EC. Cyto-sponge, as a minimally invasive method for microbiota sampling, demonstrated superior performance by yielding higher quantities of microbial DNA and capturing a comparable microbial profile to biopsy and brush samples, while exhibiting an enrichment of taxa from the oral cavity and stomach13. In addition, organoids have emerged as a valuable ex vivo tool for modeling esophageal homeostasis and disease, faithfully reproducing the dynamic characteristics of the esophageal epithelium. Organoids successfully recapitulate normal epithelial renewal, differentiation, and proliferation, making organoids suitable for studying disease-specific alterations in response to various pathogenic stimuli70. Co-culture models of 3D organoids and the gut microbiome have enabled the faithful characterization of the consequences of microbe-epithelial interactions71. It is clear that 3D organoid models are an ideal platform for examining host–pathogen interactions in the co-culture of the microbiome and esophageal tissue. In addition, the escalating adoption of target therapies in EC has led to accumulating evidence suggesting the potential involvement of esophageal microbiota in modulating the patient response to these treatments72,73, thus presenting a significant focus of research in this field.
Our exploration of the association between the microbiome and EC has considered various factors that can influence changes in the esophageal microbial community, including medications, immune response, dietary habits, and age. Additionally, we have investigated the oral microbiome, which is suspected to be a risk factor for EC, particularly ESCC. However, there are discrepancies in these findings, and significant gaps persist in our understanding of the etiology, pathology, and immunology of this disease. Therefore, large-scale prospective cohort studies are invaluable to monitor the longitudinal changes in the microbiome during lesion progression and intervention. The current sampling methods for esophageal flora are becoming more diverse, and the use of non-endoscopic cell sampling device, like the cyto-sponge, offers non-invasive and convenient sampling options that can facilitate further exploration of the esophageal flora.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed the analysis: Jun Li, Hongle Li.
Collected the data: Yi Li.
Contributed data or analysis tools: Xia Xue, Yi Li, Bing Wei.
Performed the analysis: Yi Li, Jun Li.
Wrote the paper: Yi Li, Jun Li.
Footnotes
↵*These authors contributed equally to this work.
- Received May 19, 2023.
- Accepted September 6, 2023.
- Copyright: © 2024, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.