Immune checkpoint blockade (ICB) has achieved durable clinical responses and has significantly improved the overall survival of cancer patients1. Among the ICB agents, programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) antibodies are used to treat various human tumors by blocking PD-1/PD-L1 signaling. Impressive response rates with low autoimmune toxicity have been reported in 20%–30% of non-selected patients1; however, most patients fail to respond to PD-1/PD-L1 blockade or acquire resistance during therapy through unknown mechanisms1. Recently, clinical data have indicated that PD-L1 expression and tumor-infiltrating lymphocyte density are associated with a better response to anti-PD-1/PD-L1 therapy1. Indeed, these findings highlight the urgent need for a better understanding of the molecular mechanisms underlying PD-L1 regulation because such knowledge may facilitate the development of alternative ICB strategies or the design of more precise and effective combinations of immune checkpoint therapies. In addition, dysregulated immune cells residing in the tumor microenvironment (TME) may contribute to increased cancer immune tolerance. Moreover, the intrinsic mechanisms involved in regulating immune cell function may be potential targets for switching from an immunosuppressive to an active status.
Long non-coding RNAs (lncRNAs) are a class of transcripts ≤ 200 nucleotides in length with no coding potential2. LncRNAs are multifunctional molecules that interact with RNA, DNA, or proteins to influence key signaling pathways in different cellular contexts, and thus lncRNAs engage in numerous physiologic and pathologic processes, including processed involving tumors2. Increasing evidence has also demonstrated the functional relevance of lncRNAs in directly controlling PD-L1 expression and regulating immune cell activities within the TME to affect the clinical outcome of PD-1/PD-L1 blockade3,4.
Regulation of the PD-1/PD-L1 pathway in cancer by lncRNAs
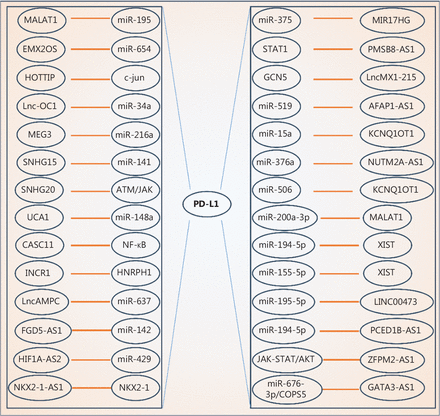
PD-L1 has been shown to be a biomarker for patient prognosis or tumor immunotherapy success5. Aberrant PD-L1 expression is frequently detected in various human cancers, so significant efforts have been made to understand how PD-L1 is regulated in cancer cells. PD-L1 levels are subjected to diverse regulatory mechanisms at the genetic, epigenetic, and post-translational levels6. Previous studies have provided additional markers to predict ICB efficacy, strategies to improve PD-L1 detection in tumor samples, and avenues to modulate PD-L1 activity. More recently, PD-L1 regulation by lncRNAs has been reported (Table 1, Figure 1). Notably, most of these lncRNAs, including LINC00473, FGD5-AS1, and MALAT1, regulate PD-L1 expression primarily via sponging microRNAs (miRNAs)7,8. LncRNAs localize to the nucleus, cytoplasm, or exosomes, and thus are able to regulate gene expression in many different ways. For example, NKX2-1-AS1, ZFPM2-AS1, and lncMX1-215 regulate tumor immunity by mediating PD-L1 expression binding to different partners7,8. Different lncRNAs have been shown to regulate PD-L1 expression at different levels3; however, most of these studies have focused on miRNA sponging function of lncRNAs. Until recently, regulation of PD-L1 expression mediated by lncRNAs has been reported to occur at the translational level, independent of miRNAs.
LncRNAs regulate PD-L1 expression. LncRNAs are involved in controlling PD-L1 expression by interacting with different miRNAs and factors or by regulating various signaling pathways.
Overview the roles of lncRNAs in regulating PD-L1 expression
The correlation between lncRNAs and PD-L1 expression highlights the predictive and targetable value of lncRNAs in the response to and efficacy of PD-1/PD-L1 blockade immunotherapy; however, lncRNAs possess cell type-specific features, with relatively low levels of expression. To date, most lncRNA studies have relied heavily on deep RNA sequencing, in which various cell types contribute to an average signal, limiting the discovery of cell type-specific lncRNA functions9 because PD-L1 is expressed in different cell types, including cancer cells, immune-related cells, endothelial cells, and fibroblasts. PD-L1 expression, particularly in cancer and immune-related cells, is closely associated with ICB efficacy. Single-cell RNA-sequencing is a potential solution to overcome these limitations, despite the lack of annotations for low abundance, yet cell type-specific lncRNAs9. Furthermore, combining several lncRNAs with PD-L1 for prediction and targeting may be more conducive to benefit anti-PD-1/PD-L1 immunotherapy.
LncRNAs regulate innate immune cells in the tumor immune microenvironment (TIME)
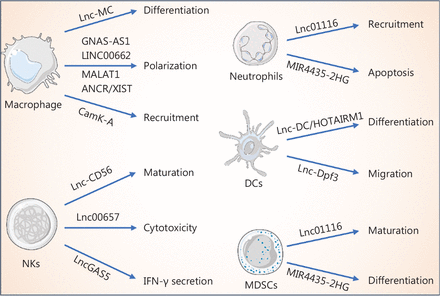
One of the main goals of immune checkpoint inhibitors is to boost effector T cell activity. A positive correlation between T cell infiltration and effector T cell activity in the TME has been demonstrated; however, T cells are not autonomous with respect to anti-tumor functions. The triggering and maintenance of anti-tumor T cell responses depend on innate immune responses10. The innate immune system includes various cell types, such as myeloid lineage cells, natural killer cells (NKs), and dendritic cells (DCs)10. When a tumor is recognized by innate immune cells, these cells launch an adaptive immune response that results in tumor regression mediated by the killing effect of cytotoxic T cells. LncRNAs have recently been reported to be associated with the differentiation, polarization, recruitment, apoptosis, maturation, and cytotoxicity of innate immune cells by regulating functional gene expression11 (Table 2, Figure 2). Neutrophils derived from myeloid progenitor cells participate in innate and adaptive responses. Different polarization states have contradictory effects on anti-tumor immune responses. N1-type neutrophils kill tumor cells via antibody-dependent cell-mediated cytotoxicity after the initiation of an adaptive response, while N2-type cells have been reported to inhibit T cell activation by inducing arginase 1 (ARG1) and ROS. It has been demonstrated that lncRNAs, such as Lnc01116 and Mir4453-2HG, participate in neutrophil recruitment and apoptosis11,12. Macrophages are phagocytic cells that are critical effector cells in innate immunity. In addition to phagocytic activity, macrophages contribute to the initiation of adaptive immune responses by releasing cytokines and chemokines. Macrophage types include M1 and M2 phenotypes. M1-type macrophages suppress tumor growth, whereas M2-type macrophages promote tumor progression. Several studies have shown that lncRNAs, such as Lnc-MC, GNAS-AS1, LINC00662, MALAT1, ANCR, XIST, and CamK-A, have important roles in macrophage differentiation, polarization, and recruitment11. NKs are innate lymphoid cells with cytotoxic effector functions. These cells are characteristically efficient at killing malignant cells with no MHC restrictions and at limiting tumor progression. Lnc-CD56, Lnc00657, and LncGAS5 have been reported to regulate the maturation and cytotoxicity of NKs11. DCs are professional antigen-presenting cells and are responsible for recognizing danger-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) to initiate specific T cell responses and are therefore important in promoting protective immunity. Within the cancer framework, DCs carry tumor antigens, which are presented to T cells to activate anti-tumor responses. The antigen-presenting activity of DCs is mediated by maturation and migration capacities. Lnc-DC, HOTAIRM1, and Lnc-Dpf3 have been reported to participate in the differentiation and migration of DCs11. Myeloid-derived suppressor cells (MDSCs) have emerged as important contributors to tumor progression and have been implicated in limiting the effects of cancer immunotherapy. MDSCs consist of two subgroups of cells, polymorphonuclear MDSCs, which are characteristically similar to neutrophils, and monocytic MDSCs, which are phenotypically and morphologically similar to monocytes. AK036396 and RNCR3 regulate MDSC maturation and differentiation11. Therefore, lncRNAs are expressed in different types of immune cells and have roles in regulating cancer immunity by modulating immune cell functions. This characteristic should be considered during the development of lncRNA-targeted therapies.
LncRNAs regulate innate immune cells. LncRNAs mediate macrophage differentiation and regulate macrophage polarization and recruitment. In addition, the recruitment and apoptosis of neutrophils are regulated by lncRNAs. Furthermore, lncRNAs mediate the maturation, cytotoxicity, and IFN-γ secretion of NKs. In addition to the regulatory functions in innate immune cells described above, lncRNAs control dendritic cell differentiation, migration, and MDSC maturation and differentiation.
Overview of lncRNA role in regulating innate cells in the TIME
LncRNAs regulate adaptive immune cells in the TIME
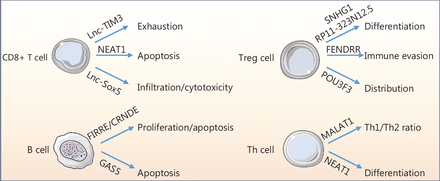
The adaptive immune system, including T and B cells, is responsible for initiating anti-tumor immune responses and immunologic memory2. T cells are the major immune cells involved in adaptive immunity2 and are classified into multiple subgroups based on immune functions and immunomodulatory roles. Importantly, the diverse subtypes of T cells in the TME have a strong correlation with the prognostic significance of PD-1/PD-L1 blockade therapy. Recently, lncRNAs have been shown to be functionally relevant in adaptive immunity2. A rapidly accumulating body of evidence suggests that lncRNAs from cancer or immune cells in the TME have many decisive and indispensable functions in T and B cell viability, migration, proliferation, and cytotoxicity14 (Table 3, Figure 3). CD8+ T cells are the major tumor-killing effector T cells in the tumor bed. Numerous studies over the last few years have revealed that lncRNAs, such as NEAT1 and lnc-TIM3, have crucial roles in CD8+ T cell apoptosis and exhaustion14. In addition to viability and migration, CD8+ T cell cytotoxic function determines the anti-tumor effects. Lnc-Sox5 promotes tumor immune evasion by inhibiting CD8+ T cell infiltration and cytotoxicity14. T helper cells are critical for activation of the anti-tumor response alone of via stimulation of CD8+ T cells. The lncRNAs MALAT1 and NEAT1, participate in the differentiation of naive T helper cells into specialized populations of effector cells, including anti-tumorigenic Th1, pro-tumorigenic Th2, and Th17 cells11. Regulatory T cells (Tregs) comprise another pro-tumorigenic T helper cell population. The differentiation and distribution of Tregs regulate pro-tumorigenic functions. LncRNAs (SNHG1, POU3F3, and RP11-323N12.5) regulate cancer cell immune evasion by mediating the differentiation and distribution of Tregs14. The adaptive immune system also relies on B cell function. In recent years, the role of T cells in anti-tumor immunity has been extensively studied; however, research on B cells is still in its infancy. B cells have been shown to differentiate into plasma cells after stimulation with tumor antigens. Plasma cells then migrate to the tumor site and secrete various cytokines and antibodies to amplify the anti-tumor immune response via phagocytosis and complement activation. LncRNAs, such as FIRRE, GAS5, and CRNDE, affect B cell proliferation and apoptosis via the regulation of gene expression11. In fact, approximately 30% of coding genes are transcribed during B cell differentiation; however, the number of aberrant lncRNAs is far greater than the number of coding genes. This finding emphasizes the importance of lncRNAs in B cell function.
LncRNAs regulate adaptive immune cells. LncRNAs mediate CD8+ T cell exhaustion, apoptosis, infiltration, and cytotoxicity. In addition, lncRNAs control the ratio of Th1-to-Th2 cells and regulate Th cell differentiation. Furthermore, lncRNAs mediate the differentiation, distribution, and immune evasion of Tregs. Additionally, lncRNAs regulate B-cell proliferation and apoptosis.
Overview of the lncRNA role in regulating adaptive cells in the TIME
RNA therapeutic prospects
As described above, lncRNAs expressed in cancer or immune cells contribute to immune evasion. LncRNAs represent promising molecules that may serve as prognostic biomarkers or potential therapeutic targets in combination with anti-PD-1/PD-L1 antibodies. Over the past decade, substantial efforts have been made towards the clinical application of RNA-based therapeutics. Approval of an mRNA-based vaccine against SARS-CoV-2 demonstrated the clinical feasibility, safety, and efficacy of such therapeutics16. These findings have paved the way for the application of RNA therapeutics in cancer immunotherapy. Initially, RNA was considered unsuitable as a therapeutic target because RNA is resistant to degradation by ribonucleases17. In addition, conventional RNA agents may induce toxicity and immune responses, thereby limiting the development of RNA-based treatment strategies17. Many shortcomings have been identified with these treatment strategies, including the chemical modification of the RNA structure in parallel with RNA delivery techniques and protection technology applications18. Compared to conventional drug formats, RNA therapeutics has a regulatory role in cancer progression, mainly by controlling the levels of target protein expression. The most prevalent RNA therapeutics can be divided into two main groups: coding RNA in vitro transcribed-messenger RNA (iVT-mRNA); and self-amplifying RNA (SAM) and non-coding RNAs (ncRNAs), including lncRNA, circular RNA (circRNA), miRNA, small interfering RNA (siRNA), and antisense oligonucleotides (ASOs)16. Although these ncRNAs cannot be translated into proteins to perform oncogenic or suppressor functions as can coding RNAs, ncRNAs participate in cancer cell biology to varying degrees. Hence, ncRNA-based approaches are an attractive field in cancer medicine research and provide a rationale for clinical application in cancer treatment. In particular, because RNA therapeutics have immunomodulatory functions, RNA molecules are highly desirable for immunotherapy19.
LncRNA-based cancer immunotherapy
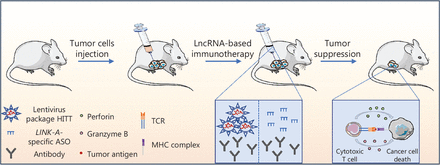
Coding RNA-based therapies, represented by mRNA therapeutics, have emerged as powerful tools for the treatment of various human diseases, especially malignant tumors. By targeting multiple tumor-specific neoantigens or tumor suppressor genes and by introducing mRNA-based cancer vaccines, mRNA-based treatments have been shown to elicit passive anti-tumor immune responses by activating the innate and adaptive immune systems. NcRNAs constitute > 90% of the RNAs in the human genome; however, most of the > 50,000 known ncRNAs have only been discovered in the past 10 years. Some of the ncRNAs have been shown to have essential roles in modulating the TIME. SiRNAs and miRNAs bind to targets via base-complementary binding to effectively and specifically regulate target gene expression. These RNAs can affect CD8+ T cells, Treg infiltration, macrophage differentiation, and DC presentation. More recently, ASO-based therapeutic strategies have been characterized by targeting non-degradable RNAs using other RNA silencing approaches. Many ASOs have shown promising immunomodulatory roles, such as regulating T cell differentiation, macrophage polarization, and MDSC polarization, by manipulating the expression of their target genes. CircRNAs have been reported to sponge miRNA to regulate MDSC migration and NK and CD8+ T cell cytotoxic activity in the TME. Although the findings between tumor and immune cells indicate the potential of lncRNAs as advanced therapeutic targets, the combination of immunotherapy and lncRNA-based targeted therapies is still in its infancy. The reason for this situation may be multifaceted, such as the length of lncRNAs being > 200 bp, which is not suitable for RNA delivery systems. In addition, RNAs > 200 bp in length are more susceptible to degradation by ubiquitous ribonucleases. Therefore, further narrowing down the functional lncRNA domain sequence with the results of basic research will contribute to promote the feasibility of lncRNA-based immunotherapy. Recently, an anti-tumor and pro-immunity lncRNA, HIF-1α inhibitor at translation level (HITT), was identified20. HITT coordinates with regulator of G protein signaling 2 (RGS2) to bind to the 5′-untranslated region of PD-L1, which leads to reduced PD-L1 translation and the subsequent activation of CD8+ T cells20. The functional fragment in HITT was narrowed down from 2,000 nt to 62 nt (1,080–1,142 nt). Notably, third-generation lentivirus packaging systems have been approved for the treatment of human diseases owing to the high levels of safety and efficiency21. Interestingly, a significant anti-tumor immunotherapy effect was achieved by the intra-tumor injection of the lentivirus expressing the functional sequence of HITT. The combination of anti-PD-1 therapy and lentivirus particles expressing the HITT fragment produced a synergistic anti-tumor effect when compared to monotherapy with an anti-PD-1 antibody20 (Figure 4). In addition, preclinical studies have shown that lncRNAs, which play roles in modulating cancer immunity through diverse mechanisms, such as reducing antigen presentation or MHC molecule expression, can be used as potential targets to improve ICB efficiency. For example, Hu et al.22 showed that ASOs specifically targeting LINK-A, an immunosuppressive lncRNA, are able to inhibit LINK-A expression and sensitize tumor cells to ICB therapy by increasing tumor cell MHC expression (Figure 4). These data indicated the potential application of lncRNAs in the treatment of cancer by boosting T cell immunity or enhancing the efficiency of PD-1/PD-L1 blockade.
LncRNA-based cancer immunotherapy prospects. The in situ injection of ICB combined with HITT expression lentivirus particles or the combination of an immune checkpoint inhibitor with a LINK-A-specific ASO produced an obvious anti-tumor effect by promoting the tumor cell killing activity of cytotoxic T cells.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Wrote the manuscript: Qingyu Lin.
Review and editing: Ying Hu.
Project administration: Ying Hu.
Validation: Qingyu Lin, Jiaqi Zhu, Ying Hu.
Acknowledgements
We thank Dr. Xingwen Wang at Institute of Technology for graphic assistance during the preparation of this manuscript.
- Received May 8, 2023.
- Accepted July 14, 2023.
- Copyright: © 2023, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.