Abstract
Objective: Cancer is one of the most common diagnoses in elderly patients. Of all types of abdominal cancer, colorectal cancer (CRC) is undoubtedly the most frequent. Median age at diagnosis is approximately 70 years old worldwide. Due to the multiple comorbidities affecting elderly people, frailty evaluation is very important in order to avoid over- or under-treatment. This pilot study was designed to investigate the variables capable of predicting the long-term risk of mortality and living situation after surgery for CRC.
Methods: Patients with 70 years old and older undergoing elective surgery for CRC were prospectively enrolled in the study. The patients were preoperatively screened using 11 internationally-validated-frailty-assessment tests. The endpoints of the study were long-term mortality and living situation. The data were analyzed using univariate Cox proportional-hazard regression analysis to verify the predictive value of score indices in order to identify possible risk factors.
Results: Forty-six patients were studied. The median follow-up time after surgery was 4.6 years (range, 2.9-5.7 years) and no patients were lost to follow-up. The overall mortality rate was 39%. Four of the patients who survived (4/28, 14%) lost their functional autonomy. The preoperative impaired Timed Up and Go (TUG), Eastern Cooperative Group Performance Status (ECOG PS), Instrumental Activities of Daily Living (IADLs), Vulnerable Elders Survey (VES-13) scoring systems were significantly associated with increased long term mortality risk.
Conclusion: Simplified frailty-assessing tools should be routinely used in elderly cancer patients before treatment in order to stratify patient risk. The TUG, ECOG-PS, IADLs and VES-13 scoring systems are potentially able to predict long-term mortality and disability. Additional studies will be needed to confirm the preliminary data in order to improve management strategies for oncogeriatric surgical patients.
keywords
Introduction
A third of new breast cancer diagnoses occur in women aged over 70 years in the UK1. The number of women affected will increase over time due to the aging population2. The prevalence of co-morbidity and frailty increases with age, resulting in deaths from other causes exceeding breast cancer mortality in older women with breast cancer3,4. Additionally, tolerance of some breast cancer therapies is reduced in this age group5,6. The majority (about 90%) of older women are diagnosed with estrogen receptor (ER) positive disease3. Older patients with operable breast cancer may be offered alternative treatment modalities, such as primary endocrine therapy (PET)7,8, where ER positive disease is treated with endocrine therapy alone without surgery. Non-operative management of older breast cancer patients accounts for up to 40% of treatment in the UK9.
PET gained popularity in the 1980s for the management of older women after tamoxifen was shown to be effective in this setting10 and a succession of randomised controlled trials comparing its efficacy with surgery followed. A subsequent Cochrane review comparing PET with surgery in the over 70s demonstrated superior rates of local control with surgery but no difference in survival rates11. However the studies included in the review were small and flawed by modern standards because tumor ER status was not always tested and the age range of the trials included relatively young older women with no documentation of health, fitness or frailty, in fact all patients were deemed fit for surgery under general anaesthesia and therefore frail patients were excluded. A recent review of case series indicated that older frailer women tend to be treated with PET in routine practice and have inferior survival rates as would be expected due to higher other-cause mortality12. Since the trials included in the Cochrane review were conducted there have been significant increases in population life expectancy, so it is not clear that whether results of these trials are applicable in contemporary clinical practice.
Recent reports have advocated the use of PET only in the very old or frail13. Current national guidelines state that patients with operable breast cancer should be treated with surgery, and not PET, “irrespective of age” unless this is precluded by comorbidities14; whilst the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA) recommend that PET should only be offered to patients with a “short estimated life expectancy (less than 2 to 3 years), who are considered unfit for surgery… or who refuse surgery”15. However, as a large number of older women are treated with PET in UK and other countries, it is not clear that this guidance is being followed consistently.
Population cohort studies have been carried out to investigate the factors associated with non-surgical treatment of breast cancer in older women and found that increasing age, comorbidities and worsening scores on activities of daily living (ADL) were all associated with lower rates of surgery16. An analysis of English cancer registry data showed that after adjusting for disease characteristics, age and comorbidity, there remained significant variation in the rate of PET use at the hospital level17. This may reflect genuine variation in practice, which would contradict current recommendations. However, this analysis could not account for other potential confounding factors such as frailty and cognitive function, which would be expected to influence treatment decision making. There are also limitations concerning data quality such as missing data due to the retrospective and routine nature of data collection. A further confounding factor is the influence of the patient’s preference for one or other option if they are given a choice.
The study presented here aims to identify patient and tumor factors associated with treatment allocation in the older UK breast cancer population, including data on functional status, cognitive function and comorbidities, as well as disease characteristics. A planned interim analysis was undertaken using prospectively collected patient data from a large multicenter UK cohort study.
Materials and methods
Study design
This study is part of a prospective observational cohort study of women aged over 70 years diagnosed with operable primary breast cancer in 51 UK breast cancer units between February 2013 and June 2015. Recruitment for this trial is ongoing, and results presented here represent an interim analysis of the first 1,447 patients. Data were collected on patient and tumor characteristics, and treatment type. Data collection time points for the study are at baseline (prior to any treatment being administered), 6 weeks post-treatment, 6 months post treatment, 12 months post-treatment, 18 months post-treatment, and 24 months post-treatment. Analyses are restricted to data collected up to 6 months post-treatment as the outcome of interest was primary treatment allocation.
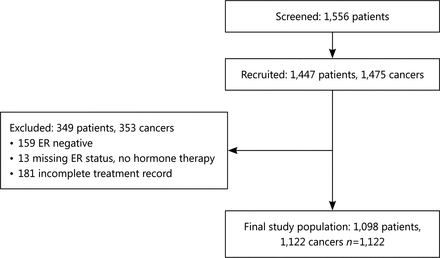
Analyses are also restricted to the subgroup of patients with operable ER positive disease at diagnosis. Variables used within the analysis are shown in Table 1. Patients with missing treatment data or with known ER negative disease were excluded from the analysis. Patients with missing ER status who did not receive endocrine therapy were assumed to be ER negative and also excluded from the analysis. Bilateral cancers are counted individually. Figure 1 shows the baseline features of the study population.
Determining the study population.
Characteristics of the study population
Statistical analysis
Primary treatment was dichotomised as “surgery” or “PET” according to whether or not the patient received surgery to the primary tumor. Simple logistic regression was used to investigate associations between complete covariates and treatment. The joint effect of patient level factors on the probability of surgical treatment was assessed using multivariable logistic regression.
Variables with missing data cannot be included in standard regression models, but excluding cases with missing values (complete case analysis) can produce biased results where data is not missing completely at random, as well as reducing statistical precision. Missing data on disease characteristics and comorbidity was handled using the method of multiple imputation by chained equations (MICE)18. Missing covariate values were imputed using predictive mean matching19, and all variables included in the main analysis were incorporated into the imputation models. Twenty-five completed datasets were used for subsequent analysis. Results of the regression models for each dataset were combined using the standard methods described by Rubin20. Covariates with over 50% missing data were not included in the regression models.
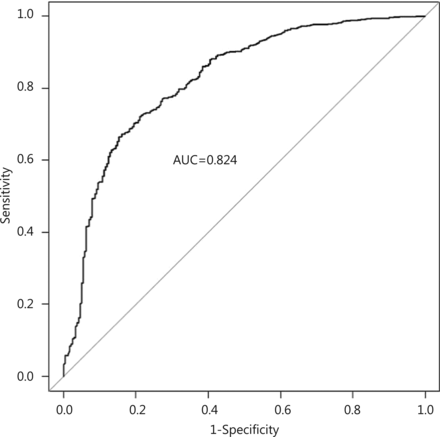
The performance of the regression model was assessed by considering the receiver-operating characteristic (ROC) curve of the model, along with its corresponding area under the curve (AUC) statistic. This is a measure of the ability of the model to discriminate between individuals treated with surgery and PET21. The AUC can range from 0.5 to 1, where a value of 1 indicates perfect discrimination, and a value of 0.5 less indicates a model which performs no better than random chance.
All analyses were conducted using the open source statistical programming language R (version 3.0.1). Multiple imputation was implemented using the “mi” package (version 1.0).
Ethics and research governance
The study protocols were approved by the UK National Research Ethics Committee (12/LO/1808) and institutional approvals were granted at each site. Written informed consent was obtained from the patient, or patient consultee in the case of patients with cognitive impairment.
Results
Data were available on 1,122 ER positive breast cancers in 1,098 patients with a treatment record of either primary surgery with adjuvant therapy as deemed appropriate by the treating clinician (“surgery”) or endocrine therapy without surgery (“PET”). Characteristics of the study population can be seen in Table 1.
About 78% of the study population were treated surgically, with the remainder being treated with PET. The patients treated with surgery were younger overall than those treated with PET (Table 1). The imputation algorithm converged successfully, and exploratory analysis of the distributions of the imputed values did not raise concerns about their validity or plausibility.
The multivariable logistic regression model is shown in Table 2. Increased age at diagnosis, increasing levels of comorbidity, large tumor size (5 cm or more) and dependence in one or more ADL categories are all strongly associated with non-surgical treatment (Table 2). The ROC curve shows that the model discriminates well between individuals treated with PET and surgery, with the AUC statistic equal to 0.824 (95% CI, 0.792-0.855) (Figure 2).
Multivariable logistic regression of surgical treatment vs. non-surgical treatment, conditional on individual patient characteristics
Receiver-operator characteristic (ROC) curve for the multivariable logistic regression model (Table 2), with area under the curve statistic (AUC).
Discussion
Of the 1,122 ER positive operable breast cancers enrolled into the study to date, 78% were treated with surgery. Age was confirmed as one of the most important factors in determining treatment in older women with operable ER positive breast cancer, with increasing age associated with higher rates of PET, even accounting for other patient and disease characteristics. This is consistent with other studies7,22,23 despite current UK guidelines, which state that patients should be treated with surgery and appropriate adjuvant therapy “irrespective of age”14.
Increasing age was associated with increasing rates of comorbidity and multi-morbidity24, this in turn may reduce the survival benefit of more aggressive breast cancer therapies, such as surgery4. It was therefore unsurprising that higher levels of co-morbidity in the study population are strongly associated with lower levels of surgical treatment, however, this does not account for the effect seen with age.
Functional dependence in one or more of the ADL categories was strongly associated with non-surgical treatment in this study. Rates of functional dependence also increase with age and have been shown to affect treatment in a small population of older breast cancer patients22. Functional dependence has been associated with increased rates of post-operative complications and longer hospital stays in elderly cancer patients25, possibly explaining why it has an impact on treatment allocation here.
Additionally, large tumor size (5 cm or more) was also associated with higher rates of PET. Tumors of this size usually mandate a mastectomy26, and so these findings may represent patients and clinicians trying to avoid more major surgery.
The rate of surgical treatment was higher in this study than some of the published figures for the UK, which range from 55%-83%8,16,17,27-29. This may result from a recruitment bias as patients within this study had to be recruited prior to commencing treatment, which was logistically more difficult with patients treated with PET as there is a shorter time period between diagnosis and treatment compared with surgery. Another possible bias could be that clinicians may be more likely to ‘follow the guidelines’ knowing that their data were being captured. This will ultimately result in an under-representation of PET patients, and may explain why Lavelle and colleagues found a similar rate of 83% having surgical treatment in their prospective cohort study16.
This study is limited by missing data, particularly with relation to ADL and MMSE, which is due, in part of patients opting out of completing the questionnaires. We have used multiple imputation to account for these missing data as it is less prone to bias than other commonly used methods to account for missing data. However, whilst exploratory analysis of the imputed data suggested that the values were plausible, it is not possible to verify the extent to which the distribution of the imputed data accurately represents that of the missing values. By using 25 imputations, uncertainty due to missing data is incorporated into the estimated effects of covariates on the probability of surgery, which mitigates against any small biases due to problems with the imputation model.
The findings of this study confirm existing research showing that age remains a significant predictor of non-surgical treatment after adjusting for disease characteristics and the underlying health status of the patient. However, it remains unclear as to whether or not current practice resulted in sub-optimal outcomes. An attempt to compare outcomes under PET and surgery with adjuvant endocrine therapy using a randomised clinical trial failed due to lack of recruitment, which was explained by an unwillingness on behalf of both patients and clinicians to choose treatment at random30. As a result, observational research is needed to investigate the effects of non-surgical treatment in this population. As recruitment and follow up of this cohort matures, it will be possible to investigate the relationship of treatment and individual characteristics with outcomes such as survival and treatment failure. These data will be crucial in the development of evidence based guidance for patients and clinicians to help target treatments effectively.
Acknowledgements
The authors would like to thank all the members of the Bridging the Age Gap in Breast Cancer Trial Management Group: Charlene Martin, Jenny Newhouse, Rosie Cooper, Alistair Ring, Stephen Walters, Tim Stephenson, Kirsty Pemberton, Tim Chater, Jaqui Gath, Lisa Caldon, Kate Lifford, Kate Brain. In addition, the authors would like to acknowledge the Principal Investigators and staff working at each of the recruiting sites: Ms. J Dicks (Barnsley), Ms. C Rogers (Doncaster and Bassetlaw), Ms. S Edwards (Milton Keynes), Mr. R Vijh (North Lincolnshire and Goole), Miss M Kaushik (Leicester), Ms. S Ashworth (Burnley), Ms. A Norton (Harrogate), Ms. R Nasr (York), Mr. C Holcolmbe (Royal Liverpool), Mr. A Nejum (Airedale), Ms. S Hartup (Leeds), Mr. R Linford (Bradford), Ms. C Morris (Cardiff and Vale), Ms. H Sweetland (Cardiff), Ms. A Williams (Nevill Hall Hospital), Mr. S Parker (Coventry), Mr. A Modi (Grantham), Ms. E Gullaksen (Hull), Ms. L Whisker (Nottingham), Mr. A Haq (Southport), Miss V Pope (Leighton), Ms. Karen Brooks (Royal Marsden), Ms. J Forkes (Cheltenham), Mr. M Douek (Guy’s and St Thomas), Ms. C Osborne (Dorset County), Ms. S Miles-Dua (Broomfield), Ms. S Buckley (Pinderfields), Ms. K Kirby (Southmead, Bristol), Miss I Azmy (Chesterfield), Mr. S Nicol (Rotherham), Miss S Seetharam (Dartford), Ms. C Osborne (Yeovil), Mr. S Joshi (Croyden), Ms. A Chilvers (North Tees), Mr. Cheema (James Cook South Tees), Ms. L Rivett (Luton and Dunstable), Miss R Ainsworth (Great Weston). This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Grant No. RP-PG-1209-10071). The views expressed are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR or the Department of Health.
Footnotes
Conflict of interest statement No potential conflicts of interest are disclosed.
- Received September 18, 2015.
- Accepted November 4, 2015.
- Copyright: © 2015, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.