Abstract
Objective: Patients with head and neck cancer often suffer from malnutrition. This study aims to investigate the influence of body mass index (BMI) on the prognosis of laryngeal squamous cell carcinoma (LSCC).
Methods: A total of 473 patients with LSCC initially treated at Sun Yat-sen University Cancer Center between January 2005 and July 2009 were retrospectively reviewed. Survival analysis was performed by the Kaplan-Meier method and Cox regression model.
Results: Low BMI before treatment was significantly associated with poor overall survival in patients with LSCC (P<0.001). BMI was an independent prognostic factor for patients with LSCC.
Conclusion: Leanness before treatment was associated with poor prognosis in patients with LSCC. Good nutritional status is favorable to improve survival in patients with LSCC.
keywords
Introduction
Head and neck cancer is the sixth most common cancer worldwide, and the most common malignancy of the head and neck is laryngeal squamous cell carcinoma (LSCC)1,2. This disease often involves male patients older than 50 years old. LSCC seriously affects the life quality of patients, and the 5-year survival is about 60% regardless of stage, treatment strategy, and tumor location1,3.
Several factors are associated with the prognosis of LSCC, including smoking and alcohol consumption, human papillomavirus and Epstein-Barr virus infections, radiation, and heredity1,2. Patients with head and neck cancer often suffer from malnutrition. Nutritional status has been reported to be associated with risk4 and survival in several types of head and neck cancer, such as oral5–7 and pharyngeal cancers8. Understanding the prognostic value of nutritional status in patients with LSCC bears clinical significance.
Body mass index (BMI), a measure of relative weight based on the mass and height of an individual, is widely used to evaluate nutritional status. BMI has been identified as a possible risk factor for several cancers. Several studies have suggested that high BMI indicates a significantly better prognosis than low BMI for patients with oral cavity and oropharyngeal cancer5,6,8,9, whereas other researchers reported conflicting results7. However, the influence of BMI on the prognosis of head and neck cancer, including LSCC, remains controversial. In the present study, we investigated the prognostic influence of BMI on LSCC.
Materials and methods
Patients and follow up
We retrospectively reviewed 473 patients diagnosed with LSCC and initially treated at Sun Yat-sen University Cancer Center (SYSUCC) between January 2005 and July 2009. The patients consisted of 456 males (96%) and 17 females (4%). Their ages ranged from 24 to 86 years (median, 59 years). The clinical characteristics of the patients are summarized in Table 1. No patient had distant metastasis prior to treatment. Among the 473 patients, 430 underwent surgery, 17 received chemotherapy and radiation treatment after surgery, 32 received radiotherapy, and 21 received chemotherapy after surgery. Surgical margins were free of disease.
Association of BMI status with the clinical characteristics of patients with LSCC
Of the 43 patients who did not undergo surgery, 11 received radiation treatment, 22 underwent chemotherapy, and 10 underwent both chemotherapy and radiation treatment.
The survival time was defined as the period from the date of the initial treatment to the date of death or final follow up (July 2014). This study followed the Declaration of Helsinki on medical protocol and ethics, and it was approved by the Institutional Review Board at SYSUCC. Written informed consent was obtained from all patients for the publication of this study.
Criteria for nutritional status
The nutritional status of the patients was classified by BMI in accordance with Asian-specific BMI cutoff points as follows: overweight (≥25 kg/m2), normal weight (≥18.5 and <25 kg/m2), or underweight (<18.5 kg/m2)10. The BMI data were obtained before treatment.
Statistical analysis
The Chi-square test and Kruskal-Wallis H test were used for bivariate analysis. Kaplan-Meier and log-rank tests were used for survival analysis. Multivariate Cox regression analysis was performed for significant variables found using univariate analysis. SPSS 16.0 was used for all analyses. P<0.05 was considered statistically significant.
Results
Patient characteristics and bivariate analysis
A total of 473 patients participated in this study. The clinicopathological characteristics of the patients are summarized in Table 1. Among the 473 patients, 83 (17%) were underweight, 296 (63%) were of normal weight, and 94 (20%) were overweight based on their BMI. The groups of patients with different BMI statuses showed similar distributions of host and tumor factors, including age, gender, smoking and alcohol intake, and pathology grade. Patients with low BMI showed a high incidence of advanced T stage (47.0% vs. 32.5% vs. 24.5%, P=0.029) and N stage (25.3% vs. 11.8% vs. 9.6%, P<0.001; Table 1).
All patients were divided into the survival and non-survival groups. The survival group consisted of 332 patients (70%), and the non-survival group comprised 141 patients (30%). The bivariate analysis of factors between the groups is presented in Table 2. The two groups showed significant differences in age, smoking and alcohol intake, T stage, N stage, pathology grade, treatment strategy, tumor location, and BMI status. However, no significant difference was found in gender between the two groups.
Bivariate descriptive analysis of patients with LSCC
Prognostic value of BMI
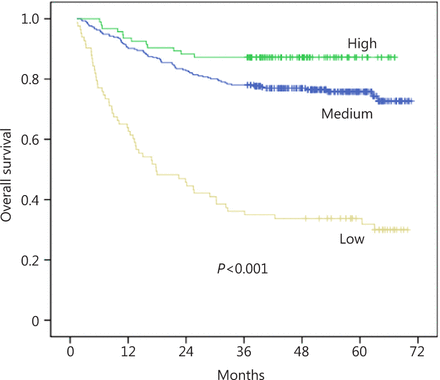
The overall 5-year survival rate of these patients was 72.3%. Figure 1 reveals that the underweight patients had the lowest survival, whereas the overweight patients had the best survival (P<0.001). The 5-year overall survival rates of the overweight group, normal weight group, and underweight group were 87.2%, 78.0%, and 34.9%, respectively. The median survival time of the underweight group was 29.6 months.
Kaplan-Meier curves for overall survival according to BMI.
The Cox proportional hazards model was used to verify whether the BMI status and other variables are independent prognostic factors for LSCC patients. Univariate analysis showed that age, history of smoking and alcohol intake, T stage, N stage, pathology grade, tumor location, treatment strategy, and BMI were associated with survival in patients with LSCC. Multivariate analysis of these variables showed that age, history of smoking and alcohol intake, T stage, N stage, pathology grade, treatment strategy, and BMI were independent prognostic factors for patients with LSCC, and low BMI was significantly associated with poor prognosis (Table 3).
Univariate and multivariate Cox regression analysis for overall survival in patients with LSCC
Discussion
Our study demonstrated that BMI was significantly associated with the prognosis of LSCC, and low BMI before treatment predicted poor prognosis in patients with LSCC.
Malnutruitional status is reportedly associated with poor prognosis in several types of cancers4-8,11. In these studies, leanness was found to be associated with increased risk for head and neck cancer regardless of the smoking and drinking status, and it was reported as an early marker of oral and pharyngeal cancer4-8. For example, oral cancer patients with a BMI <22.8 kg/m2 have poor prognosis, and weight loss can predict poor prognosis in recurrent oral and oropharyngeal carcinoma5,6. A previous retrospective analysis reported that patients with head and neck cancer and preoperative weight loss greater than 5% show poor outcome11. Notably, a study by Iyengar et al.7 indicated that obesity is an independent predictor of increased risk of death for patients with early-stage oral tongue cancer. These conflicting results may be due to an inadequate sample size and inconsistent BMI cutoff values. These studies also found various tumor sites in the oral cavity, oropharynx, and larynx with various types of histopathology. In the current study, a total of 473 patients diagnosed with LSCC were analyzed, and the patients were classified according to Asian-specific BMI cutoff values. We found that low BMI was significantly associated with poor survival in LSCC, which was in agreement with previous data.
Malnutrition commonly occurs in patients with head and neck cancer, particularly in laryngeal cancer, which seriously affects the quality of life and nutritional status of the patient. Thus, weight loss before treatment is associated with poor prognosis. In China, underweight patients are associated with low income and education. These patients are less likely to receive proper treatment at an early stage. In addition, good nutritional status can improve survival by strengthening immunity and helping patients develop a high tolerance for extended therapeutic periods. However, our conclusion requires further demonstration. We found that patients with low BMI showed an increased incidence of advanced T and N stages, which may also contribute to decreased survival in patients with low BMI.
The present study demonstrated that patients over 60 years old exhibited low survival rates. Aging patients with oral tongue cancer have been reported to have high disease-specific mortality12. This finding suggested increased vulnerability to deterioration of nutritional or physical status among old patients. Heavy tobacco and alcohol consumption were determined to be adverse prognostic factors in patients with head and neck cancer, including laryngeal cancer13. Another study identified heavy alcohol consumption as a risk factor for malnutrition14. Postoperative complications have also been reported to be associated with poor prognosis in patients with oral cancer15. The present study found similar factors that could contribute to the advanced disease stage or poor general condition, both of which could increase the risk of developing complications. Developing complications would inevitably delay the administration of standard therapy, leading to recurrence or distal metastasis. Recurrence or distal metastasis may negatively affect initial treatment. Consequently, patients with low BMI have poor prognosis5.
The molecular and cellular mechanisms by which BMI affects the prognosis of cancer are complicated and unclear. Several studies have shown that a specific enteral nutrition formula containing n-3 fatty acid can prevent the deterioration of nutritional status during chemoradiation therapy for head and neck cancer; triglycerides have also been reported to promote cancer cell proliferation, and this anti-apoptotic activity is probably due to the generation of reactive oxygen species and oxidative stress, which leads to DNA damage16,17. Levels of adipokine, leptin, and adiponectin influence obesity and several signal transduction pathways involved in cell survival18. Another study reported that obesity induces insulin-resistance through the accumulation of macrophages that secrete inflammatory mediators, such as tumor necrosis factor-α, interleukin-6, and prostaglandin E219. The insulin receptor activating signaling pathways are also associated with invasion and metastasis of malignant tumors20. However, the mechanisms require further study.
Conclusion
Our study proved that BMI was an independent prognostic factor for patients with LSCC, and low BMI before treatment could predict poor prognosis for patients with LSCC. Good nutritional status could improve survival in patients with LSCC. However, our study was limited by the retrospective design. Thus, further studies are necessary to verify the correlation between BMI and the prognosis of LSCC.
Acknowledgements
This work was supported by the Science and Technology Planning Project of Guangdong Province, China (Grant No. 2010B031600237).
Footnotes
↵* These authors have contributed equally to this work.
Conflict of interest statement No potential conflicts of interest are disclosed.
- Received June 5, 2015.
- Accepted September 21, 2015.
- Copyright: © 2015, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.