Abstract
OBJECTIVE To investigate the protein expression of HBME–1, CD15 and p53 in thyroid carcinoma and in bengin thyroid diseases and to evaluate their value in diagnosis of thyroid disease.
METHODS Sixty–one cases of thyroid carcinoma and 27 cases of different kinds of benign thyroid diseases were studied by immunohistochemical methods.
RESUTLS All cases of differentiated thyroid carcinoma were positive for HBME–1, 35.7% positive for CD15 and only 14.3% positive for p53. Twenty–seven cases of benign thyroid lesions were absolutely negative for p53, 7.4% of which were CD15 positive and 25.9% of which were HBME–1 positive.
CONCLUSION HBME–1 and CD15 may be helpful to assist differential diagnosis of thyroid carcinoma from benign thyroid diseases.
keywords
At the present time, the diagnosis of papillary thyroid carcinoma is mainly based on nuclear features[1,2]. Unfortunately, these features can be also found in some benign lesions of the thyroid such as hyperplasia, thyroiditis and even in normal glands [3]. Invasion of the capsule and invasion into the veins in or beyond the capsule represent the diagnostic criteria for carcinoma in a follicular thyroid neoplasm, but sometimes it is very difficult to confirm whether there is capsule invasion. How can we draw a distinction between the malignant and the benign in this situation? It seems reasonable that the biological changes caused by the alterations of gene expression should be earlier than the morphologic alterations of cells during tumor generation and progression, so it may be possible that studies of unusual gene expression may guide us diagnostically in the future. Several studies have shown that some antibodies such as HBME–1[4], CD15[4], p53[5] and S–100[6], reacted with thyroid carcinoma but not with benign thyroid epithelium. Here we studied a series of thyroid tumors and non-neoplastic lesions by immunohistochemical methods and evaluated the biological significance and potential diagnostic value of these findings.
MATERIALS AND METHODS
Formalin fixed, paraffin–embedded tissue from 88 surgically resected thyroid specimens were obtained from the Department of Pathology of Peking University. These cases were classified histologically according to the published criteria [1,2]. There were 61 cases of thyroid carcinoma including 47 cases of papillary carcinoma, among them 11 cases were microcarcinoma (less than 10 mm in diameter), 9 cases of follicular carcinoma, 3 cases of medullary carcinoma, 1 case of squamous carcinoma and 1 case of undifferentiated carcinoma. Twenty–seven cases were benign thyroid disease including 8 cases of adenoma, 9 nodular goiter with papillary hyperplasia, 7 Grave’s disease with papillary hyperplasia and 3 lymphocytic thyroiditis.
All specimens were fixed in 10% neutral–buffered formalin and embedded in serial 3–4mm paraffin blocks; 4–μm–thick sections were stained with hematoxylin and eosin for histological examination. Representative sections were then immunostained to localize the expression of HBME–1, CD15, p53 and S–100.
Immunohistochemical staining was done using the labeled streptavidin biotin (LSAB) method (DAKO, Glostrup, Denmark, LSAB kit) for HBME–1, CD15, p53 (DAKO, Glostrup, Denmark; monoclonal, dilution 1:50) and S–100 antiserum (DAKO, Glostrup, Denmark; polyclonal, dilution 1:50). In the negative control the primary antibody was omitted.
Each antibody was tested with retrieval antigen using a microwave as the source of heat exposure for 15 min. Sections were incubated in the primary antibody for 24 hr at 37°C and then incubated with biotinylated secondary antibody for 30 min at room temperature, followed by peroxidase-conjugate streptavidin. Staining was visualised using DAB, washing in water, and counterstaining with haematoxylin.
Membranous staining with or without cytoplasmic staining was scored as appropriate for HBME–1 and CD15. Nuclear staining was scored as appropriate for p53. Nuclear staining with or without cytoplasmic staining was scored as appropriate for S–100. HBME–1, CD15, p53 and S–100 were assigned as positive, based on the commonly used clinical cut-off points of 10% of tumor cell staining, regardless of the intensity.
RESULTS
All results are summarized in Table 1.
Results of immunohistochemical examinations in thyroid lesions
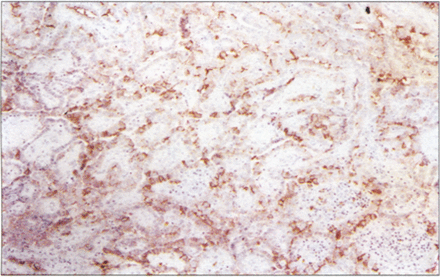
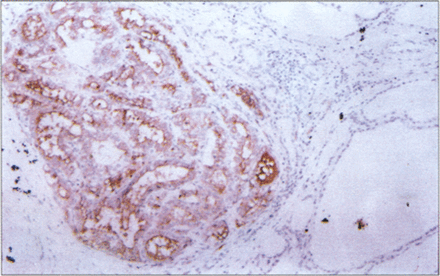
In this study, HBME–1 was found in a significant proportion of thyroid malignancies. All cases of differentiated thyroid carcinoma (papillary carcinoma and follicular carcinoma) had more than 10% of positive tumor cells (Fig. 1, 2), while poorly differentiated thyroid carcinoma (spindle cell carcinoma) and medullary carcinoma was absolutly negative. Most cases of papillary carcinoma showed strong and widespread HBME–1 expression, including 11 cases of microcarcinoma (Fig.3). Follicular variants showed expression similar to that of the tumors with a papillary appearance. HBME–1 expression often appeared as luminal and lateral cell membranous staining. In 50% of the cases there was general cytoplasmic reactivity as well. The surrounding thyroid tissue was typically negative for HBME–1.
Papillary thyroid carcinoma is widespread HBME–1–positive. LSAB immunoperoxidase with haematoxylin counterstain, ×200.
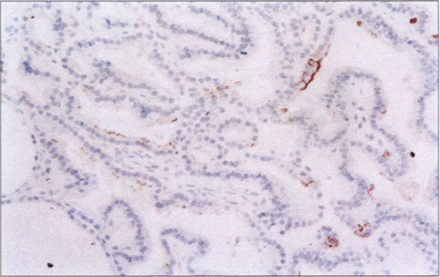
Follicular thyroid carcinoma is diffusely HBME–1–positive. LSAB immunoperoxidase with haematoxylin counterstain, ×200.
Microcarcinoma is strongly HBME–1 positive, in sharp contrast to the surrounding thyroid tissue. LSAB immunoperoxidase with haematoxylin counterstain, ×100.
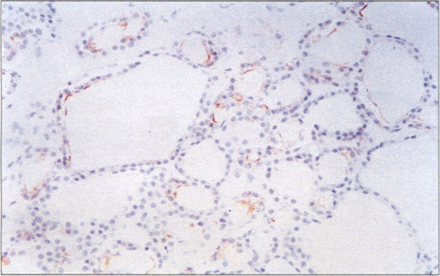
Nodular goiters were generally HBME–1 negative. However, HBME–1 positive cells were seen in 6/9 of cases with papillary hyperplasia. HBME–1 reactivity was seen in focal areas with complex papillary structure and nuclear changes (Fig.4). Papillary lesions with coarse chromatin or PTC–like nuclei (nuclear grooves and clearing) displayed positive reactivity to HBME–1. The more complex the papillary structure was, the stronger the reactivity. In our study, none of the seven cases of Grave’s disease with papillary hyperplasia showed any HBME–1 reactivity.
An encapsulated thyroid nodule with extensive papillary architecture is focally HB–1–positive. LSAB immunoperoxidase with haematoxylin counterstain, ×200.
Follicular adenomas were typically HBME–1 negative. However, three cases of adenoma showed HBME–1 reactivity (more than 10% of tumor cells). One was atypical adenoma; the reactivity approached the level that was seen in thyroid carcinoma. The other two were Hürthle–cell lesions which showed only cytoplasmic reactivity similar to that shown by one case of lymphocytic thyroiditis.
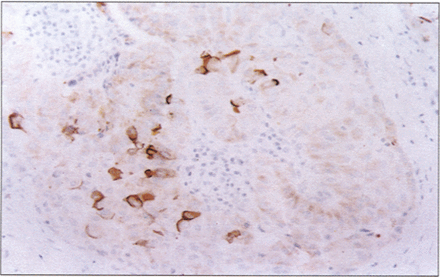
The localization of CD15 immunoreactivity in thyroid carcinoma was generally similar to that of HBME–1, although the number of positive cases and the number of tumor cells in positive cases were much smaller than those for HBME–1 (Fig. 5). Only 35.7% of cases of differentiated thyroid carcinoma had more than 10% positive tumor cells. CD15 positive cells were less than 50% of all tumor cells in every CD15 positive case. There was no difference for CD15 expression between papillary and follicular carcinoma. Seventeen total cases of papillary carcinoma were CD15 positive, three of them were classified as encapsulated variants; and only three had cervical nodal metastases at the time of diagnosis. The case classified as diffuse sclerosis variant showed CD15 negativity. The surrounding thyroid tissue was absolutely negative for CD-15.
Papillary thyroid carcinoma is strongly CD15–positive. LSAB immunoperoxidase with haematoxylin counterstain, ×400.
Although HBME–1–positive cells were seen in some cases of nodular goiter with papillary hyperplasia, all the cases including nodular goiter and Grave’s disease with benign papillary hyperplasia were typically negative for CD15. The reactivity for CD15 in adenoma was the same as that for HBME–1.
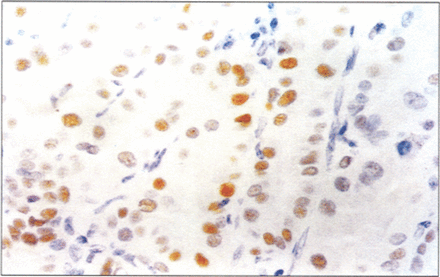
Overall, p53 expression confined to the nucleus was identified in 14.9% (7/47) of papillary carcinoma (Fig. 6) and in 11.1% (1/9) of follicular carcinoma. Seven cases of papillary carcinoma all were p53 positive, one of which was the case of diffuse–sclerosis variant and four had cervical nodal metastases at the time of diagnosis. The two cases classified as encapsulated variants were p53 negative. No immunohistochemical positivity was found in adjacent non-neoplastic thyroid tissues or in benign lesions.
Papillary thyroid carcinoma is p53–positive. LSAB immunoperoxidase with haematoxylin counterstain, ×400.
In our study, not only thyroid carcinoma but also benign thyroid lesions such as thyroid adenoma, nodular goiter and thyroiditis displayed positive reactivity to S–100.
DISCUSSION
The differential diagnosis between benign and malignant lesions is essentially based on cytohistological interpretation. Sometimes the “histologically benign–look” tumor was in fact biologically aggressive. Although the underlying mechanism leading to carcinogenesis is not well known, we can say on the basis of modem cell biology that the morphologic change is just the result of alteration of gene expression. Thus immunohistochemical determinations may be helpful in the diagnosis of histologically difficult thyroid lesions.
HBME–1 is a monoclonal antibody directed against an unknown epitope on mesothelial cells. Several studies[4,7,8] have shown its utility as a marker in the identification of malignant follicular epithelial lesions of the thyroid. Our study demonstrated diffuse HBME–1 expression in 100% of differentiated thyroid carcinoma including eleven cases of microcarcinoma. Thus diffuse HBME–1 positivity may have diagnositc value in evaluation of thyroid lesions. Papillary carcinoma is the most common malignant neoplasm of the thyroid gland. In many cases, there are some minute satellite lesions outside of the main tumor mass; sometimes these foci of tumor may be overlooked in H&E staining sections. We found that they were highlighted with HBME–1 immunostaining. So it would be helpful to do immunostaining for HBME–1 before one makes a diagnosis of microcarcinoma. If there are some small foci of tumor cells outside of the main tumor less than 10 mm in size, the diagnosis will be a usual papillary carcinoma rather than a microcarcinoma.
CD15 expression is widely used as a diagnostic marker of Hodgkin’s cells and in the differential diagnostic panel for evaluation of possible mesothelioma [9,10]. A few of previous studies have explored the expression of CD15 in thyroid carcinoma[4]. Unfortunately, our data showed that only 36.2% of patients with papillary thyroid carcinoma were CD15 positive, which limited the use of CD15 as a differential indicator. The previous study has suggested that CD15 reactivity might be an unfavorable prognostic indicator in thyroid papillary cancer[11]. Our results showed that CD15 positivity does not necessarily mean unfavorable prognosis.
In our study, CD15 was shown to be a relatively specific marker in differentiation of papillary thyroid lesions. HBME–1–positive cells were seen in some cases of nodular goiter with papillary hyperplasia, while all the cases of nodular goiter with papillary hyperplasia were typically negative for CD15. So if a papillary lesion is CD15 positive, it most probably is malignant and other criteria of malignancy such as nuclear features should be looked for carefully.
Invasion of the capsule and invasion into veins represent the diagnostic criteria for carcinoma in a follicular thyroid neoplasm, but sometimes it is very difficult to confirm whether there is true invasion or not. The combination of HBME–1 and CD15 immunostaining will be a powerful tool for the diagnosis of follicular thyroid lesions. HBME–1 reactivity associating with CD15 reactivity indicates a possibility of malignancy and a more careful search should be done for other features of malignancy.
The p53 gene mutation is found in about half of all tumors. In our study, immunohistochemistry was performed to identify mutations of the p53 gene in thyroid lesions. Results showed that the incidence of p53 reactivity was low(14.3%). So p53 immunostaining has little significance in differential diagnosis of thyroid tumors. This study supported the concept that the p53 mutation status was a prognosticator of tumor behavior in thyroid carcinoma [5,12]. It seems that overexpression of p53 is not a responsible factor for the oncogenesis itself, but rather that it plays a crucial role in aggressive subtypes of thyroid carcinoma.
Our data showed that S–100 immunostaining would not be useful in differential diagnosis of thyroid tumors. This result is in disagreement with that of Kilicarslan et al[6].
In summary, malignant thyroid tumors show significantly greater HBME–1 and CD15 reactivity than benign lesions. Although the biochemical bases of HBME–1 and CD15 are not very clear yet, these antibodies may highlight part of the molecular changes in cells related to malignant transformation. Along with careful histological evaluation, this panel of two immunohistochemical markers provides a useful and objective means in diagnosing difficult thyroid lesions. Diffuse HBME–1 positivity indicates a malignancy, which is confirmed by CD15 reactivity. However, caution must be exercised in the interpretation, because the results in benign (usually negative) vs malignant (usually positive) lesions may overlap.
- Received February 2, 2004.
- Accepted March 22, 2004.
- Copyright © 2004 by Tianjin Medical University Cancer Institute & Hospital and Springer