Abstract
Objective: The human cluster of differentiation (CD)300A, a type-I transmembrane protein with immunoreceptor tyrosine-based inhibitory motifs, was investigated as a potential immune checkpoint for human natural killer (NK) cells targeting hematologic malignancies (HMs).
Methods: We implemented a stimulation system involving the CD300A ligand, phosphatidylserine (PS), exposed to the outer surface of malignant cells. Additionally, we utilized CD300A overexpression, a CD300A blocking system, and a xenotransplantation model to evaluate the impact of CD300A on NK cell efficacy against HMs in in vitro and in vivo settings. Furthermore, we explored the association between CD300A and HM progression in patients.
Results: Our findings indicated that PS hampers the function of NK cells. Increased CD300A expression inhibited HM lysis by NK cells. CD300A overexpression shortened the survival of HM-xenografted mice by impairing transplanted NK cells. Blocking PS–CD300A signals with antibodies significantly amplified the expression of lysis function-related proteins and effector cytokines in NK cells, thereby augmenting the ability to lyse HMs. Clinically, heightened CD300A expression correlated with shorter survival and an “exhausted” phenotype of intratumoral NK cells in patients with HMs or solid tumors.
Conclusions: These results propose CD300A as a potential target for invigorating NK cell-based treatments against HMs.
keywords
Introduction
Cancer profoundly impacts human life1, and in recent years immune checkpoint therapy (ICT) has made remarkable strides in cancer treatment. ICT has significantly transformed the clinical outcomes of cancer patients, providing enduring clinical benefits, including cures in some cases2. Several immune-checkpoint inhibitors have gained approval for treating solid tumors3–8. Furthermore, ICT has demonstrated therapeutic efficacy against hematologic malignancies (HMs)9,10.
However, the application of ICT faces formidable challenges, such as a low overall response rate (especially in cancers with a low mutation burden), the emergence of immune-related adverse events, and the development of primary and adaptive resistance2,11. Notably, in HMs constrained by immune dysfunction and a lack of suitable targets, the efficacy of ICT is notably limited, as predominantly observed in specific subtypes of lymphomas12,13. To address these challenges, the exploration of new immune checkpoints becomes imperative.
The human cluster of differentiation (CD)300A, a type-I transmembrane protein, stands out as a potential ICT target. CD300A is characterized by a single immunoglobulin (Ig)V-like extracellular domain (validated by crystallography) and an extended membrane-proximal region rich in proline, serine, and threonine that links the Ig and transmembrane domains14,15. The long cytoplasmic tail of CD300A contains immunoreceptor tyrosine-based inhibitory motifs that exerts its inhibitory function by recruiting Src homology region 2 domain-containing phosphatase-1 (Shp-1) in the cytoplasm16,17. Several studies have shown that CD300A, which is expressed in various leukocytes, has a role in regulating functions, such as activation, proliferation, differentiation, and immune function18–23.
Phosphatidylserine (PS) has emerged as a CD300A ligand24,25. PS resides on the inner side of the normal cell membrane. PS often exhibits spontaneous exposure in tumor cells due to dysregulated flippases and scramblases26. PS serves as a global inhibitory signal in cancer27. The presence of PS on tumor cells and associated vesicles is linked to mechanisms of tumor escape28. Antibody (e.g., bavituximab) targeting PS has demonstrated efficacy in tumor therapy29. Thus, CD300A may function as an immune checkpoint and blockade of the CD300A–PS axis could be a promising addition to immune checkpoint-based cancer immunotherapy.
HMs represent sensitive tumor types in which natural killer (NK) cells exert a lytic function30. Investigating the impact of CD300A on the anti-HM function of NK cells is essential. Additionally, determining if CD300A blockade using monoclonal antibodies enhances the anti-HM capacity of NK cells is of paramount importance.
Herein we report that PS exerts an inhibitory effect on NK cell function, and this inhibitory function is dependent on CD300A. CD300A inhibits NK cell lysis against HM cells in vitro and this effect is partially suppressed by blocking the PS signal. Furthermore, CD300A suppresses the capacity of NK cells to act against HMs. Blockade of CD300A with the recombinant monoclonal antibody, TX4931, enhances NK cell lytic function. Additionally, increased infiltration of CD300A-positive lymphocytes within tumors is observed compared to peri-tumoral tissues. Analysis of The Cancer Genome Atlas (TCGA) database also revealed that high expression of CD300A is associated with shorter survival and a more “exhausted” phenotype of NK cells. Taken together, these findings indicate that CD300A functions as an immune checkpoint on NK cells, negatively regulating lytic function by “sensing” the PS signal. Blockade of CD300A with antibodies may be a therapeutic approach in HM treatment.
Materials and methods
Ethical approval of the study protocol
The study protocol received approval from the Ethics Committee of the University of Science and Technology of China (Approval No. USTCACUC23060122060). Additionally, the use of clinical samples in the study was sanctioned by the Ethics Committee of Shanghai Outdo Biotech (Shanghai, China). Clinical samples were processed in a de-identified manner.
Mice, cell lines, and reagents
NOD/ShiLtJGpt-Prkdcem26Cd52Il2rgem26Cd22/Gpt (NCG) 8-week-old male mice were procured from GemPharmatech (Nanjing, China) and maintained in specific pathogen-free conditions following the National Guidelines for Animal Usage in Research, as promulgated by the Chinese government at the University of Science and Technology of China.
Cell lines (K562, U937, NCI-H929, HL-60, HepG2, and HEK293T) were obtained from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). NK-92 cells were sourced from the American Type Culture Collection (Manassas, VA, USA). The ExpiCHO™ expression medium was acquired from Thermo Scientific (Waltham, MA, USA). HL-60, K562, U937, and NCI-H929 cells were maintained in RPMI-1640 medium (VivaCell, Beijing, China) with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 U/mL) at 37 °C in 5% CO2. NK-92 cells were cultured in alpha-minimum essential medium (Thermo Scientific) containing 12.5% fetal bovine serum, 12.5% horse serum, human recombinant interleukin-2 (100 U/mL), inositol (10 mM), 2-mercaptoethanol (0.1 mM), folic acid (0.02 mM), penicillin (100 U/mL), and streptomycin (100 U/mL) at 37 °C in 5% CO2. HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium (VivaCell, Shanghai, China) with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 U/mL) at 37 °C in 5% CO2. ExpiCHO™ cells were cultured in ExpiCHO expression medium according to the manufacturer’s instructions.
Flow cytometry
CellTrace™ Violet (CTV) was obtained from Invitrogen (C34557; Carlsbad, CA, USA) for flow cytometry. 7-Aminoactinomycin (7-AAD; 559925), annexin V (556420), and anti-CD45 (557833 and 564047), anti-CD56 (744218), anti-CD4 (557871), anti-CD8 (557746), anti-CD69 (557745), anti-NKG2D (557940), anti-PD-1 (557860 and 560795), anti-LAG3 (565720), anti-perforin (563393), anti-IFN-γ (559327), anti-TNF (563418), anti-CD69 (557745), anti-NKp30 (558408) and anti-mouse (mIgG) antibodies were obtained from BD Biosciences (San Jose, CA, USA). Additional antibodies [anti-CD56 (362538), anti-CD3 (300328), anti-CD244 (329512), anti-IFN-γ (506504), anti-TNF (502909), anti-granzyme B (515406), anti-CD107a (328632), anti-TIGIT (372714), anti-CTLA-4 (369610), anti-NKp44 (325116), and anti-hIgG Fc (409320)] were obtained from BioLegend (San Diego, CA, USA). Anti-CD300A (FAB2640G and FAB2640R) and anti-NKG2A antibodies (FAB1095P) were obtained from R&D Systems (Minneapolis, MN, USA).
For staining surface markers, peripheral blood mononuclear cells (PBMCs) were washed and incubated for 30 min at 4 °C in 1× PBS with 10% mouse serum (Future Biotechnology, Guangzhou, China). The cells were then stained with antibodies for 30 min at 4 °C in the dark. For staining CD107a and intracellular cytokines, PBMCs were stimulated with different concentrations of PS (Aladdin, Shanghai, China) for 18 h. After PS stimulation, PBMCs were stimulated for 4 h with 30 ng/mL phorbol 12-myristate 13-acetate [(PMA), Sigma, Darmstadt, Germany] and 1 μM ionomycin (Sigma) in the presence of 2.5 μg/mL monensin (eBioscience, Waltham, MA, USA). After stimulation, PBMCs were stained for surface markers, washed, fixed, and permeabilized with fixation buffer according to the manufacturer’s instructions (eBioscience). Fixed cells were incubated for 30 min at 4 °C in 1 × PBS with 10% mouse serum, then stained with antibodies to perforin, granzyme B, IFN-γ, and TNF. All samples were acquired on a flow cytometer (LSR-II or Celesta; BD Biosciences) and analyzed using FlowJo (TreeStar, Ashland, OR, USA).
Construction of a CD300A-overexpressed NK-92 cell line
A CD300A expression vector was constructed using recombinant-DNA technology based on the pLVX-IRES-zsGreen plasmid. The vector was introduced into NK-92 cells (activated with 800 U/mL IL-2) by lentivirus in the presence of polybrene (8 μg/mL). GFP+ cells were subsequently isolated by flow sorting.
Expression and purification of TX49
The TX49 expression vector was constructed through recombinant-DNA technology based on the pTT5 plasmid. TX49 was purified from the supernatants of transfected ExpiFectamine™ CHO cells using the ExpiFectamine™ CHO Transfection Kit (Thermo Scientific). The supernatants were affinity-purified with protein A. The molecular weight and purity of TX49 were determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE).
Binding assay
To assess the binding capacity of the recombinant TX49 monoclonal antibody, CD300A-overexpressed or control NK-92 cells were incubated with TX49 (10 μg/mL) for 30 min at 4 °C. After washing, NK-92 cells were stained with anti-mIgG antibodies for 30 min at 4 °C. Subsequently, the proportion of positively stained cells was determined using flow cytometry.
To evaluate TX49 blockade to CD300A, Jurkat cells were subjected to a 10-min incubation at 50 °C to induce apoptosis. The Jurkat cells were then incubated with recombinant human CD300A-Fc fusion protein (12449-H02H; SinoBiological, Beijing, China) for 30 min in the presence or absence of TX49 monoclonal antibody. Fusion protein binding to cells was detected using anti-hFc antibody.
Cytotoxicity assay
K562, U937, HL-60, or NCI-H929 cells (1 × 104) were labeled with CTV and co-incubated with PBMCs or NK-92 cells at various effector:target (E:T) cell ratios in the presence of PBS, IgG, or TX49 for 4 h. For spontaneous-death control, CTV-labeled tumor cells were cultured alone under identical conditions. Wells were harvested after 4 h and 7-AAD was added before analysis. Samples were thoroughly mixed and analyzed using flow cytometry.
Real-time cytotoxicity assays
NK cell cytotoxicity against adherent target cells was examined using a Real Time Cell Analyzer (RTCA)-MP Instrument (xCELLigence; ACEA Biosciences, San Diego, CA, USA). Target cells (2 × 104 HEK293T or HepG2) were seeded into E-Plates and NK-92 cells were seeded into E-Plates as effectors for killing. E-Plates were placed in the RTCA-MP instrument for assays at 37°C in a 5% CO2 atmosphere.
Enzyme-linked immunosorbent assays (ELISAs)
NK-92 cells were co-cultured with HEK293T cells for 72 h to measure IFN-γ secretion. The IFN-γ content in culture supernatants was subsequently detected using the Human IFN-gamma ELISA Kit (BMS228TEN; Invitrogen) following the manufacturer’s instructions.
NK-92 cell-reconstituted xenogeneic tumors
NCG mice received intravenous (iv) injections of luciferase-expressing HL-60 (HL-60-luc) tumor cells (1 × 106) on day 0, followed by NK-92-OE cells (2 × 106 iv) or control NK-92 cells (2 × 106 iv) every 7 days for a total of 2 doses starting on day 21. Tumor-bearing mice were also intraperitoneally (ip) injected with interleukin-2 (50,000 U; Jiangsu Kingsley Pharmaceuticals, Nanjing, China) every 3 days for a total of 5 doses starting on day 21.
Bioluminescence imaging
Mice were injected ip with d-luciferin (15 mg/mL; Gold Biotechnology, St. Louis, MO, USA) at 150 mg/kg. Mice were placed into an in vivo imaging system (Caliper Life Sciences, Waltham, MA, USA) when fully anesthetized by isoflurane. Luciferase expression was imaged using Spectral in vivo (PerkinElmer, Waltham, MA, USA) and calculated using Living Image (PerkinElmer).
Statement: For animal studies, tumor-bearing mice were randomized before administration.
Quantification of cytokines in cell-culture supernatants
Cytokine concentrations were analyzed from the supernatants of cytotoxicity assays using the 13-plex LEGENDplex™ Human CD8/NK Panel (Biolegend) following the manufacturer’s instructions.
Immunohistochemistry
Paraffin sections of human tumor tissues were obtained from Shanghai Outdo Biotech (Shanghai, China). These sections underwent de-waxing, rehydration, heat-induced epitope retrieval, and incubation with primary antibodies against human CD300A (PA5-113473; Invitrogen). Signals were detected using the DAB Peroxidase Substrate Kit (SK-4100; Vector Labs, Burlingame, CA, USA).
TCGA data analyses
The overall survival of patients with acute myeloid leukemia (LAML), lung squamous cell carcinoma (LUSC), brain low-grade glioma (LGG), colon adenocarcinoma (COAD), esophageal carcinoma (ESCA), and uveal melanoma (UVM) based on CD300A mRNA expression was analyzed using the TCGA database. The survival of cancer patients was analyzed using Kaplan–Meier curves by GEPIA (http://gepia.cancer-pku.cn/).
The gene-expression correlations between CD300A and gene sets for the exhausted phenotype of NK cells (NCR1, EOMES, TBX21, TIGIT, LAG3, PDCD1, NKG2A, and HAVCR2) were analyzed by GEPIA2 (http://gepia2.cancer-pku.cn/#index).
The differential expression of CD300A in NK and CD8+ T cells from tumor patients was analyzed by GEPIA2021 (http://gepia2021.cancer-pku.cn/).
Statistical analysis
Comparisons between two groups were analyzed using unpaired one- or two-tailed Student’s t-tests. Comparisons between ≥ 3 groups were analyzed using two-way analysis of variance. The Mantel–Cox test was used to analyze mouse survival. The results are presented as the mean ± SEM. A P-value < 0.05 was considered statistically significant.
Results
Treatment with the CD300A ligand, PS, impairs NK cell function
We identified CD300A expression on NK cells in human peripheral blood through flow cytometry analysis, as illustrated in Supplementary Figure S1A. Furthermore, TCGA database analysis indicated that CD300A expression in NK cells from tumor patients surpassed CD300A expression in CD8+ T cells (Supplementary Figure S1B). Additionally, the level of CD300A expression in NK cells from cancer patients was higher than healthy controls (Supplementary Figure S1C).
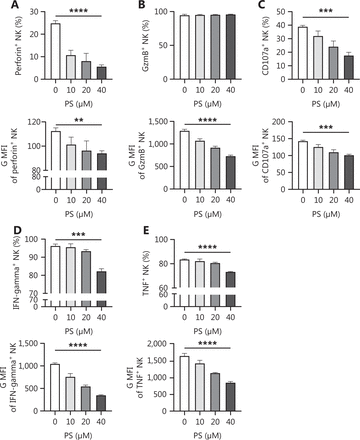
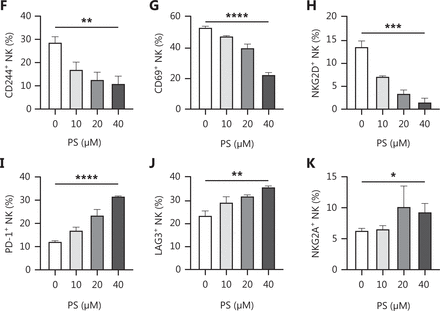
Because PS is a known CD300A ligand, we determined if PS negatively regulated NK cells from human peripheral blood. NK cells were stimulated with varying concentrations of PS for 18 h and the expression of functional proteins was assessed using flow cytometry. Treatment with PS resulted in a significant reduction in the expression of cytotoxicity-related functional molecules (perforin, granzyme B, and CD107a) and effector cytokines [interferon (IFN)-γ and tumor necrosis factor (TNF)] in NK cells. Specifically, PS treatment decreased the proportion of perforin+, CD107a+, IFN-γ+, and TNF+ NK cells in a dose-dependent fashion. Additionally, the mean fluorescence intensity of perforin, granzyme B, CD107a, IFN-γ, and TNF was significantly decreased after PS treatment in a dose-dependent manner (Figure 1A–1E). Following PS treatment, the expression of inhibitory receptors [programmed cell death protein 1 (PD-1), lymphocyte-activation gene 3 (LAG3), and NKG2A] increased, while the expression of active receptors (CD244, CD69, and NKG2D) was decreased (Figure 1F–1K). These findings collectively suggest that PS inhibits human NK cell function.
Treatment with CD300A ligand PS impairs NK cell function. NK cells were stimulated by PS (dissolved in PBS) in vitro for 18 h. The functional and surface molecules of NK cells were analyzed by flow cytometry. The percentage (top panel) and geometric MFI (bottom panel) of perforin (A), granzyme B (GzmB) (B), CD107a (C), IFN-γ (D), and TNF (E) functional molecules in NK cells are shown with statistical data. The percentage of NK cells expressing surface molecules [CD244 (F), CD69 (G), NKG2D (H), PD-1 (I), LAG3 (J), and NKG2A (K)] are shown with statistical data. Data are presented as the mean ± SEM, n = 3 per group. Statistical significance was determined using an unpaired two-tailed Student’s t-test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Enhanced PS-CD300A signals hinder the ability of NK cells to lyse HMs
We assessed the inhibitory impact of CD300A on NK cell-mediated lysis of HMs. The CD300A-negative NK-92 cell line served as the foundation for establishing a CD300A-overexpressed counterpart (designated NK-92-OE; Figure 2A). Evaluation of surface membrane proteins on NK cells revealed that NK-92-OE exhibited a significant reduction in expression of the surface activation marker, CD69, compared to NK-92-Ctrl, while the level of activation receptor (CD244, NKp30, and NKp44) expression remained consistently high (Supplementary Figure S2A–S2D). There were no noteworthy differences in the level of inhibitory receptors (PD-1, CTLA-4, and LAG3) expression (Supplementary Figure S2E-S2G). NK-92-OE expressed a higher level of TIGIT compared to NK-92-Ctrl, albeit the upregulation was not substantial (Supplementary Figure S2H). Thus, NK-92-OE demonstrated a more inhibitory phenotype and CD300A overexpression minimally affected activation and other inhibitory receptor expression.
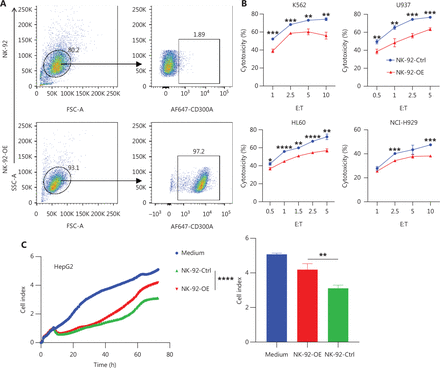
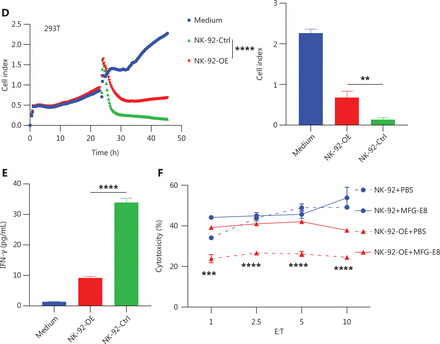
Enhanced PS-CD300A signals impair NK cell ability to lyse hematologic malignancies. (A) CD300A-expressing vectors transfected into NK-92 cells to overexpress CD300A on NK-92 cells. CD300A expression on NK-92 cells was analyzed by flow cytometry. Top panel: NK-92 cells transfected with mock vectors; bottom panel: NK-92 cells transfected with CD300A-expressing vectors. (B) NK-92 cells with or without CD300A overexpression co-cultured with K562, U937, HL60, or NCI-H929 cells in vitro at different effector-to-target cell ratios (E:T). The cytotoxicity of NK-92 cells with or without CD300A overexpression against these tumor cell lines was evaluated by flow cytometry. The percentage of lysed cells is shown. (C, D) NK-92 cells with or without CD300A overexpression evaluated for in vitro lytic activity against HepG2 cells (C) or HEK293T cells (D) by real-time cell-index measurements (xCELLigence). The cell-index curve (left) and statistical data (right) are shown. (E) NK-92 cells with or without CD300A overexpression co-cultured with HEK293T cells. IFN-γ production of co-cultured NK-92 cells was analyzed by ELISA. (F) NK-92 cells with or without CD300A overexpression co-cultured with HEK293T cells treated with MFG-E8 (10 μg/mL) or PBS for 30 min. The cytotoxicity of NK-92 cells against HEK293T cells was evaluated by flow cytometry after co-culture for 4 h. (B-F) Data are presented as the mean (C, D left) or mean ± SEM (B, E, F and C, D right), n = 3 per group. Statistical significance was determined using the unpaired two-tailed Student’s t-test (B, E, F and C, D right) or two-way ANOVA (C, D left). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
HMs encompass a spectrum of neoplastic conditions affecting hematopoietic and lymphoid tissues32. Major subtypes of HMs are categorized into acute leukemia, myeloma, and lymphoma33. We selected K562, a human chronic myeloid leukemia cell line, and HL-60, a human promyelocytic leukemia cell line, both of which belong to the leukemia subtype, as target cells. CD300A-overexpressed NK-92 cells exhibited reduced lytic capacity against both leukemia cell lines compared to control NK-92 cells (Figure 2B). NCI-H929, a human plasmacytoma myeloma cell line, and U937, a human lymphoma cell line, were selected as representative subtypes of HMs. CD300A-overexpressed NK-92 cells displayed diminished lytic capacity against these two HM subtypes (Figure 2B). Additionally, we chose a solid tumor cell line, HepG2, a human hepatocellular carcinoma cell line, as target cells, which revealed that CD300A-overexpressed NK-92 cells exhibited reduced lytic capacity against HepG2 cells (Figure 2C). Notably, HEK293T cells, which are known to exhibit spontaneous exposure of PS on the cell membrane34, showed reduced cytotoxicity against CD300A-overexpressed NK-92 cells compared to control NK-92 cells (Figure 2D).
To determine how CD300A overexpression influences NK cell-mediated lysis of tumor cells, we co-cultured NK-92 cells with K562 cells for 4 h and subsequently assessed the levels of NK cell cytotoxicity-related molecule and cytokine expression. The results indicated that compared to NK-92-Ctrl, NK-92-OE expressed lower levels of CD107a, IFN-γ, and TNF (Supplementary Figure S3A, S3D and S3E). However, the level of perforin and GzmB expression were not significantly different, remaining relatively high (Supplementary Figure S3B and S3C). Further investigation into the impact of CD300A on NK cell cytokine secretion revealed that NK-92-OE exhibited a significant reduction in IFN-γ levels in culture supernatants during co-culture with target cells, as determined by ELISAs (Figure 2E). Collectively, these findings demonstrate that CD300A negatively regulates NK cell degranulation capacity and the production and secretion of cytokines.
Lactadherin (MFG-E8), a PS-binding protein that inhibits PS signals35, prompted us to determine if CD300A hinders NK cell cytotoxicity by sensing PS signals. HEK293T cells were treated with MFG-E8 (10 μg/mL) or phosphate-buffered saline (PBS) for 30 min before cytotoxicity assays.
CD300A-overexpressed cells or control NK-92 cells were assessed for in vitro lytic activity against HEK293T cells by flow cytometry. MFG-E8 significantly enhanced the cytotoxicity of CD300A-overexpressed NK-92 cells but did not impact the cytotoxicity of control NK-92 cells (Figure 2F).
In summary, our results demonstrated that CD300A impairs NK cell-mediated lysis of HMs and inhibits NK cell lytic function by sensing its ligand, PS.
Enhanced PS-CD300A signals on NK cells hamper the ability to act against HMs in vivo
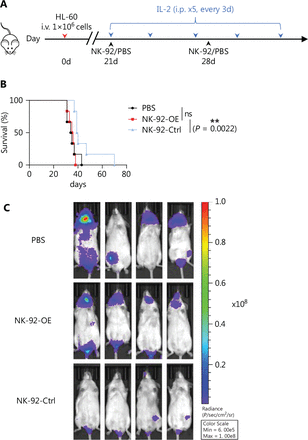
Our objective was to evaluate the impact of CD300A on the in vivo ability of NK cells to act against HMs. To this end we established a xenotransplantation model utilizing NCG mice and human promyelocytic leukemia (HL-60) cells (Figure 3A). NCG mice received an iv injection of HL-60 cells (1 × 106) on day 0. Following tumor formation, tumor-bearing mice were iv-treated with PBS, NK-92-OE cells (2 × 106), or control NK-92 cells (2 × 106). Notably, control NK-92 cells significantly improved the survival of mice compared to NK-92-OE cells (Figure 3B). In contrast, NK-92-OE cells did not significantly enhance the survival of mice compared to the PBS group (Figure 3B). Furthermore, imaging results clearly demonstrated accelerated tumor progression in the NK-92-OE group compared to the NK-92-Ctrl group. These findings strongly suggest that CD300A inhibited the anti-tumor ability of NK cells in vivo.
Enhanced PS-CD300A signals on NK cells hamper activity against hematologic malignancies in vivo. (A) Schematic of the xenotransplantation model and treatment schedule. NCG mice were injected with HL-60 tumor cells (1 × 106 iv) on day 0. Twenty-one days later, these tumor-bearing mice were treated (iv) with PBS, CD300A-overexpressing NK-92 cells (2 × 106), or control NK-92 cells (2 × 106) combined with 50,000 U of recombinant human IL-2 (ip). During treatment, tumor-bearing mice were treated with a supplemental injection of PBS, CD300A-overexpressing NK-92 cells, or control NK-92 cells (iv) at day 28, and recombinant human IL-2 was injected (ip) every 3 days. (B) Mantel-Cox survival curves of HL-60 tumor-bearing mice treated with the indicated formulation (n = 6 mice per group). Statistical significance was determined using the Mantel–Cox test. *P < 0.05. (C) In vivo bioluminescence imaging of mice 27 days after the tumor challenge described in (A). The figures displayed are representative images for each group (n = 6).
CD300A blockade enhances the ability of NK cells to lyse HMs
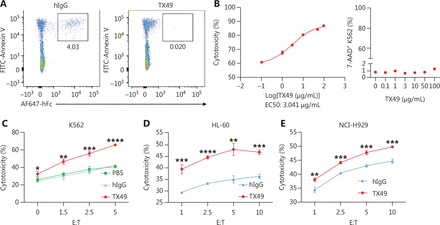
We determined whether CD300A blockade enhanced human NK cell lytic function against HMs. TX49, a monoclonal antibody targeting CD300A and blocking the interaction between CD300A and PS according to USA patent number US010519233B2, was generated and purified (Supplementary Figure S4A). The purity of TX49 exceeded 90% (Supplementary Figure S4B). Flow cytometry confirmed the binding of TX49 to NK-92-OE cells, while no binding was observed with control NK-92 cells (Supplementary Figure S4C). To validate that recombinant TX49 blocks the interaction between CD300A and PS, we assessed the ability of TX49 to inhibit the binding of CD300A-Fc fusion protein to apoptotic Jurkat cells, which present surface PS. TX49 effectively blocked CD300A binding to apoptotic Jurkat cells (Figure 4A), confirming its role as an antibody impeding the CD300A-PS interaction.
CD300A blockade enhances the ability of NK cells to lyse hematologic malignancies. (A) Detection of recombinant TX49 monoclonal antibody blocking by flow cytometry. Apoptotic Jurkat cells (highly-exposed PS) were incubated with recombinant human CD300A-Fc fusion protein for 30 min in the presence or absence of TX49 monoclonal antibodies. The binding of the CD300A-Fc fusion protein to PS on apoptotic Jurkat cells was detected using anti-hFc antibodies and flow cytometry. (B) K562 tumor cells co-cultured with CD300A-overexpressing NK-92 cells in the presence of TX49 at an E:T ratio of 5:1 for 4 h (left). The cytotoxicity of CD300A-overexpressing NK-92 cells to K562 tumor cells was determined according to the percentage of dead (7-AAD+) K562 tumor cells using flow cytometry. The percentage of dead (7-AAD+) K562 tumor cells was detected when K562 tumor cells were incubated with TX49 for 4 h without co-culturing with NK-92 cells (right). (C-E) CD300A-overexpressing NK-92 cells co-cultured with K562 (C), HL60 (D), or NCI-H929 (E) cells in the presence of TX49 or control hIgG. The cytotoxicity of CD300A-overexpressing NK-92 cells to these tumor cells was evaluated in the presence or absence of the TX49 blocking antibody. (B-E) Data are presented as the mean ± SEM, n = 3 per group. Statistical significance was determined using the unpaired two-tailed Student’s t-test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
To determine the optimal treatment dose of TX49, we assessed the cytotoxicity of NK-92 cells against K562 cells at different concentrations of TX49. TX49 treatment enhanced the cytotoxicity of NK-92-OE cells in a dose-dependent fashion, plateauing at ∼100 μg/mL (Figure 4B, left). Notably, TX49 itself did not exhibit toxicity against tumor cells (Figure 4B, right; Supplementary Figure S4D).
Our investigation extended to determining if TX49 treatment bolsters the lytic ability of NK cells against HMs. We evaluated NK-92 cell cytolytic activity against various hematologic cancer cells at different E:T ratios under TX49 treatment or control human IgG (hIgG) treatment. TX49 significantly enhanced NK-92-OE cell cytotoxicity against human chronic myeloid leukemia cells (K562), human promyelocytic leukemia cells (HL-60), and human plasmacytoma cells (NCI-H929). These results underscore that CD300A blockade using monoclonal antibodies can augment the capacity of NK cells to lyse HMs.
CD300A blockade enhances expression of NK cell lysis-function molecules
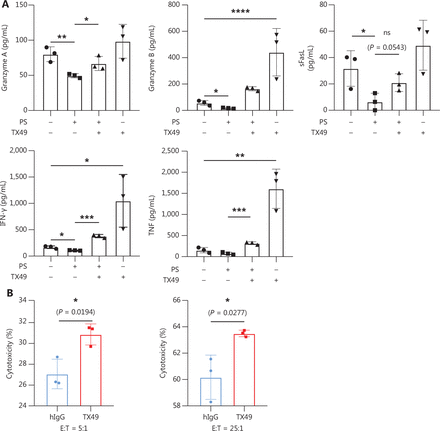
Our investigation delved into CD300A blockade augmenting the expression of lytic function-related molecules in NK cells. Co-culturing human peripheral NK cells with K562 cells at an E:T cell ratio of 10:1 in the presence or absence of PS, TX49, or a combination of PS andTX49 for 6 h, facilitated measurement of secreted lytic function-related protein levels. Secretion of granzymes, crucial effector molecules for NK cell cytotoxicity28, were significantly increased by PS-treated NK cells under TX49 treatment, with elevated levels of granzyme-A and -B (Figure 5A). Additionally, the secretion of soluble (s)FasL, a mediator of direct target-cell apoptosis by NK cells36, was mildly increased with TX49 treatment in PS-treated NK cells (Figure 5A).
CD300A blockade enhances the expression of NK cell lysis function-related molecule. (A) NK cells co-cultured with K562 cells at an E:T-cell ratio of 10:1 in the presence or absence of PS, TX49, or PS combined with TX49 for 6 h. Levels of secreted TNF, granzyme A, granzyme B, IFN-γ, and FasL in supernatants were measured by flow cytometry using the LEGENDplex™ Multi-Analyte Flow Assay Kit (Human CD8/NK panel). (B) NK cells co-cultured with K562 cells for 4 h in the presence of TX49 or control hIgG. The cytotoxicity to K562 cells was evaluated by flow cytometry. The percentage of lysed cells is also shown. (A, B) Data are presented as the mean ± SEM, n = 3 per group. Statistical significance was determined using the unpaired two-tailed Student’s t-test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Evaluation of proinflammatory cytokine secretion, which is a pivotal index of NK cell function, showed that TX49 treatment significantly increased the secretion of IFN-γ and TNF by PS-treated NK cells (Figure 5A). Furthermore, CD300A blockade with TX49 significantly enhanced the cytotoxicity of primary peripheral NK cells against K562 cells at E:T cell ratios of 5:1 and 25:1 (Figure 5B). Together, these findings demonstrated that CD300A blockade by TX49 markedly enhanced the expression of lytic function-related molecules in NK cells, thereby augmenting anti-tumor capability.
Enhanced expression of CD300A indicates shorter survival and a more inhibited NK cell phenotype in HMs and solid tumors
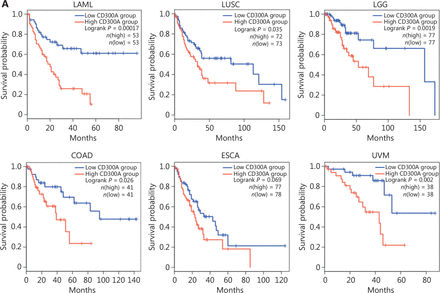
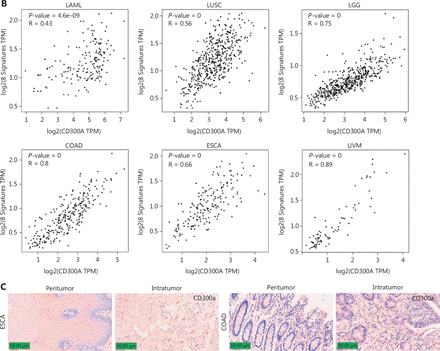
To elucidate the relationship between CD300A expression and HM progression in a clinical context, we analyzed the LAML dataset within TCGA for CD300A abundance. Notably, high CD300A expression correlated with shorter survival in LAML patients (Figure 6A). Extending this investigation to solid tumors, we analyzed datasets for patients with LUSC, brain LGG, COAD, ESCA, or UVM for CD300A abundance in TCGA. High CD300A expression correlated with shorter survival in patients with LUSC, LGG, COAD, and UVM (Figure 6A).
High expression of CD300A indicates shorter survival and a more inhibited phenotype of NK cells in hematologic and solid tumors. (A) Kaplan–Meier survival curves for patients with acute myeloid leukemia (LAML, n = 106), lung squamous cell carcinoma (LUSC, n = 145), brain low-grade glioma (LGG, n = 154), colon adenocarcinoma (COAD, n = 82), esophageal carcinoma (ESCA, n = 165), or uveal melanoma (UVM, n = 76) based on mRNA expression of CD300A in TCGA (CD300A low = blue; CD300A high = red). Data were analyzed for significance by the log-rank test. (B) Correlation between CD300A transcripts per kilobase of the exon model per million mapped reads (TPM) and the module TPM of NK inhibitory receptors (NCR1, EOMES, TBX21, TIGIT, LAG3, PDCD1, NKG2A, and HAVCR2) in tissues of patients with LAML, LUSC, LGG, COAD, ESCA, or UVM. Spearman’s correlation coefficient (R) and P-values are shown. (C) Representative immunohistochemical staining of CD300A in the peri- and intra-tumoral tissues of patients with ESCA (n = 4) or COAD (n = 5). Positive staining is indicated by the red arrows. Original magnifications: 20 ×. Scale bar = 50 μm.
To explore the relationship between CD300A expression and NK cell exhaustion within tumors, we analyzed datasets for LAML, LUSC, LGG, COAD, ESCA, and UVM in TCGA for intratumoral NK cell exhaustion. Utilizing eight specific gene sets for identifying exhausted NK cells37, our analysis revealed that high CD300A expression correlated with enhanced NK cell exhaustion in patients with LAML, LUSC, LGG, COAD, ESCA, and UVM (Figure 6B). Additionally, immunohistochemical staining of CD300A in samples collected from intra- and peri-tumoral tissues in patients with ESCA or COAD showed increased infiltration of CD300A+ cells in intra-tumoral tissues compared to peri-tumoral tissues (Figure 6C). Thus, these data suggest that high CD300A expression is associated with NK cell exhaustion.
Discussion
NK cells have a pivotal role in eliciting anti-tumor immune responses through the direct induction of apoptosis in target cells and the secretion of cytokines and chemokines to modulate the engagement of other immune cells in anti-tumor immunity30. Recent years have witnessed substantial progress in NK cell-based anti-cancer therapies38,39. Within the tumor microenvironment, however, NK cells often undergo exhaustion due to the disrupted equilibrium between inhibitory and activating receptors40. Hence, understanding the mechanisms governing NK-cell exhaustion is imperative for advancing NK cell-based immunotherapies against cancer.
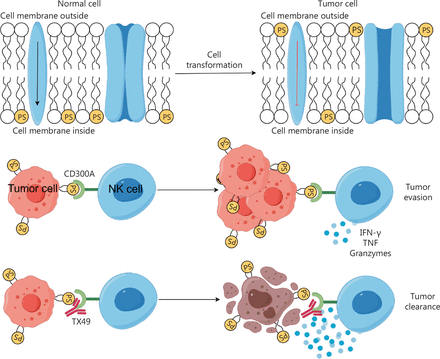
In this context we propose a mechanism for NK cell exhaustion. The initial step involves the spontaneous expression of PS on the surface of tumor cells (Figure 7). PS subsequently interacts with CD300A on the surface of NK cells, thereby conveying inhibitory signals and diminishing the capacity to secrete cytotoxic molecules and cytokines (Figure 7). Uncovering this mechanism deepens our comprehension of the tumor microenvironment and broadens our insight into how tumor cells impact NK cells.
Graphic model of the mechanisms underlying blocking CD300A enhances the anti-tumor function of NK cells. PS is located on the inner side of the normal cell membrane, while tumor cells spontaneously expose PS on the outer side of the cell membrane. The PS on tumor cells binds to CD300A expressed on the surface of NK cells, negatively regulating the anti-tumor function of NK cells and ultimately leading to tumor escape. Using antibodies to block CD300A can eliminate the negative regulatory effect of CD300A on NK cells, enhancing the anti-tumor function of NK cells. This graphic model was drawn by Figdraw.
Our study revealed that blocking the interaction between PS and CD300A on NK cell surfaces using the TX49 monoclonal antibody augmented the secretion of cytotoxic molecules and cytokines by NK cells (Figure 7). Furthermore, TX49 enhanced the ability of NK cells to lyse cells in HMs. These findings indicate that CD300A functions as a novel immune checkpoint in NK cells and the blockade of CD300A with TX49 represents a new form of NK cell-dependent ICT for HMs.
PS is situated on the inner side of the normal cell membrane. However, PS is frequently exposed spontaneously to the external surface on tumor cells26, making PS a global tumor marker27. Although PS participates in immune inhibitory responses, the mechanisms are intricate and not fully understood29. The monoclonal antibody, bavituximab, which targets PS, has demonstrated efficacy in tumor therapy29. Combining bavituximab with pembrolizumab has proven effective against gastric cancer (clinical trial number: NCT04099641) and advanced hepatocellular carcinoma (clinical trial number: NCT03519997). Our study contributes to understanding the immune suppression mediated by PS, revealing one mechanism involving inhibition of NK cell activity via CD300A. This insight supports the strategic use of monoclonal antibodies to block PS for tumor treatment and emphasizes that PS blockade can activate NK cells.
While our investigation primarily focused on the impact of CD300A on NK cell anti-tumor functions in the context of HMs, it is crucial to acknowledge that CD300A is also expressed on the surface of various myeloid cells22. Myeloid-derived suppressor cells (MDSCs) expand with tumor progression, which mediates immune suppression41 and negatively regulates NK cell functions40 and T cells42. Thus, exploring the role of CD300A on the surface of MDSCs, the influence of CD300A on tumor progression, and the effects of antibody blockade of CD300A on MDSCs within the tumor progression context presents an intriguing avenue for further research.
While our primary disease model was HMs, we demonstrated that CD300A therapy also diminished NK cell cytotoxicity against HepG2 cells. Furthermore, analyses of the TCGA database and immunohistochemical staining suggested that CD300A may serve as a prognostic indicator for solid tumors. Therefore, we hypothesize that CD300A may have a crucial role in NK cell-mediated responses against solid tumors. Subsequent experimental investigations are necessary to validate this hypothesis.
Conclusions
Our study showed that enhanced expression CD300A impaired the ability of human NK cells to lyse HMs. PS, a ligand of CD300A exposed to the outer surface of malignant cells, impairs the ability of NK cells to lyse HMs. Blockade of the PS-CD300A signal with antibody significantly enhanced lysis function-related protein and effector cytokine expression in NK cells and significantly enhanced the ability of NK cells to lyse HMs. Enhanced expression of CD300A was associated with shorter survival and a more exhausted intratumoral NK cell phenotype in patients with HMs or solid tumors. Therefore, our study revealed that CD300A is targetable and could invigorate NK cell-based treatments against different cancer types.
Supporting Information
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed the analysis: Shuangcheng Li, Zhigang Tian, Xiaohu Zheng.
Collected the data: Shuangcheng Li, Tianci Wang, Xinghui Xiao.
Contributed data or analysis tools: Xiaodong Zheng, Haoyu Sun, Rui Sun.
Performed the analysis: Shuangcheng Li, Hongdi Ma, Xiaohu Zheng.
Wrote the paper: Shuangcheng Li, Hongdi Ma, Zhigang Tian, Xiaohu Zheng.
Data availability statement
The datasets for patients with LAML (n = 106), LUSC (n = 145), LGG (n = 154), COAD (n = 82), ESCA (n = 165), or UVM (n = 76) are available in the TCGA. All other experimental data were generated by the authors and are available upon reasonable request.
- Received October 8, 2023.
- Accepted December 11, 2023.
- Copyright: © 2024, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.