Cancer immunotherapy is a preferred strategy for boosting anti-tumor immunity to eliminate malignant cells and is considered a major breakthrough in the field of cancer treatment; however, immunosuppressive cells in the tumor microenvironment (TME) pose a major obstacle to the efficacy of immunotherapy. Myeloid cells, such as tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), and immunosuppressive lymphocytes, such as regulatory T cells, are the most important immunosuppressive cells present within the TME. Such immunosuppressive cells are mainly produced by the orderly differentiation of stem and progenitor cells during bone marrow hematopoiesis. Nevertheless, recent studies have revealed that myeloid and erythroid progenitor cells (EPCs) generated by tumor-induced extramedullary hematopoiesis (EMH) have important roles in disrupting antitumor immunity and impairing the efficacy of immunotherapy. This review focuses on the mechanism underlying tumor-induced EMH, EMH-mediated immunosuppressive cell generation, and the effect on anti-tumor immunity (Figure 1).
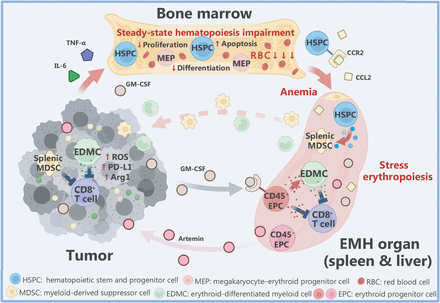
Extramedullary hematopoiesis (EMH) in cancer. Tumor-induced inflammatory factors, such as GM-CSF, TNF-α, and IL-6, damage bone marrow erythropoiesis and upregulate erythrocyte apoptosis, causing anemia and EMH. Hematopoietic progenitor cells (HSPCs) are recruited to the spleen through upregulated CCL2/CCR2 signaling and differentiate into splenic myeloid-derived suppressor cells (MDSCs) under the combined action of splenic environmental factors and endogenous to perform immunosuppressive functions. Tumor-induced EMH-generated CD45− erythroid progenitor cells (EPCs) promote tumor progression by secreting artemin. The CD45+ EPC population inhibits proliferation and immune function of CD8+ T cells by promoting overexpression of ROS, PD-L1, and Arg1, thus disrupting antitumor immunity. Furthermore, daughter cell EDMCs mediated by tumor-induced GM-CSF signals establish stronger immunosuppressive functions. (The figure was created with BioRender.com)
EMH in cancer
Mammalian hematopoietic activity begins in the yolk sac and moves to the fetal liver and spleen to produce blood cells as needed1. After birth, the bone marrow takes over the responsibility of providing and replenishing blood cells, and hematopoietic activity in the liver and spleen ceases. Hematopoietic progenitor cells (HSPCs) in the bone marrow with the capacity for self-renewal and differentiation are produced and continuously replenish all cellular components of the blood system2. Traditionally, HSPCs develop into the lymphoid, myeloid, and erythroid lineages, and the probability of developing into each lineage is relatively stable. In the physiologic state, there is no apparent intersection that differentiates between lineages, thus maintaining a lineage balance in the blood system.
EMH, however, occurs in pathologic conditions, such as infections, metabolic stress, anemia, and cancer, thus leading to impaired hematopoietic balance in the bone marrow3. Among all the pathologic states that trigger EMH, tumor-associated anemia is particularly worth noting. Tumors are associated with the release of inflammatory factors, such as GM-CSF, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6), which mainly cause inflammatory hypoferremia, iron-restricted erythropoiesis, and upregulated hemolysis, as well as decreased production and reactivity of erythropoietin (EPO)4. The mechanism underlying the release of inflammatory factors and the effects on EPO not only occurs in purely inflammatory conditions, but also due to the genetic regulatory aspects of erythropoiesis via GATA-1, GATA-2, IL-1 and interferon-γ5. Like many types of solid tumors, hematologic neoplasms, such as leukemia and multiple myeloma (MM), secrete inflammation factors that lead to disordered erythropoiesis and upregulated hemolysis. Specifically, among patients with MM, malignant plasma cells secrete monoclonal antibodies that target nearby bone tissue, which results in hypercalcemia, and together with myeloma-associated nephropathy caused by deposition of light chains in the distal convoluted tubule, a decreased production of EPO. In addition, unlike solid tumors in which infiltration is largely local before metastasis occurs, hematologic neoplasms impair the hematopoietic function directly by infiltrating the bone marrow. Indeed, abnormally proliferating, poorly differentiated cells invade and sabotage hematopoietic structures and microenvironment. Patients with hematologic neoplasms are also at high risk for anemia, but the underlying mechanisms are slightly different6. Because bone marrow hematopoiesis disorders lead to upregulation of erythrocyte apoptosis and phagocytosis, erythrocyte production declines rapidly and anemia occurs. Due to changes in the bone marrow microenvironment and the induction of cytokines which exist in anemia, the release of HSPCs from the bone marrow to the peripheral blood is much higher than in the physiologic state, and circulating HSPCs are then recruited to extramedullary organs, such as the spleen and liver7. When combined with changes in the local microenvironment, including hypoxia, a tumor-mediated systemic immune response, and large amounts of soluble factors, EMH niches form and activate extramedullary erythropoiesis3,8. EMH initiates erythropoiesis to compensate for the loss of bone marrow function when impaired bone marrow hematopoietic function is unable to produce sufficient blood cells; however, as a compensatory mechanism, EMH can only slightly compensate for bone marrow hematopoiesis dysfunction, which causes continuous progression and leads to EMH organ swelling. In most cases, EMH does not effectively alleviate the severity of tumor-associated anemia9,10. Although tumors promote EMH, the mechanism underlying EMH-mediated immunosuppressive cell generation and the role in anti-tumor immunity remain poorly understood.
Splenic HSPC-mediated tumor-promoting myelopoiesis disrupts anti-tumor immunity
Recently, EMH-induced tumor-promoting myelopoiesis has been reported in the spleen of tumor-bearing mice, suggesting that the spleen is not only a reservoir of immune cells but also has a unique and important function in producing immunosuppressive myeloid cells, which are essential for tumor-induced anti-tumor immune tolerance. Using five transplanted or genetic tumor models, a previous study showed that in response to tumor-induced splenic EMH, splenic stromal cells of tumor-bearing mice recruited Linlow/−Sca-1+c-Kithigh (LSK) HSPC subpopulations from the peripheral circulation to the spleen via the CCL2/CCR2 axis11. Then, under the “education” of soluble factors, such as IL-6 produced by splenic stromal cells and GM-CSF produced by CCR2-positive splenic LSK HSPCs, the myeloid commitment of HSPCs is synergistically driven to differentiate into highly immunosuppressive myeloid cells, which then inhibit T cell proliferation and antigen-specific cytotoxic activity. Researchers have specifically targeted splenic HSPCs with low-dose sorafenib, a c-kit inhibitor, to restrain splenic EMH while maintaining the physiologic function of the spleen. This treatment significantly induced apoptosis and inhibited proliferation of splenic HSPCs by reducing endogenous GM-CSF expression. Moreover, low-dose sorafenib treatment inhibited the suppressive effect of tumor MDSCs on T cell proliferation and cytotoxicity and synergistically enhanced the therapeutic efficacy of PD-L1 blockade.
Further studies demonstrated that most splenic LSK HSPCs with EMH showed signs of a strong endoplasmic reticulum stress response, including the activation of PKR-like endoplasmic reticulum kinase (PERK)12. HSPCs are then reprogrammed into committed MDSC precursors in the spleen via the PERK-ATF4-C/EBPβ signaling pathway. Similar to the splenic HSPC-targeted strategy of low-dose sorafenib, spleen-targeted PERK blockade restrains the immunosuppressive function of MDSCs and reshapes the tumor microenvironment, thus restoring anti-tumor immunity impaired by EMH and improving the efficacy of immunotherapy. This finding suggests that targeting EMH may be a novel strategy to enhance immunotherapy.
In addition, cancer-induced splenic accumulation of HSPCs has been observed in patients with different types of solid tumors, and patients with lower frequencies of CD133+ (a marker for human HSPCs) progenitors in the spleen had significantly longer survival than patients with higher frequencies11. These clinical results indicate that tumor-promoting myelopoiesis caused by splenic EMH has a significant negative effect on tumor patients and that CD133 can be used as a marker to predict the prognosis of patients with tumors.
Tumor-associated EMH-induced erythroid-derived immunosuppressive cells impair antitumor immunity
In addition to the large accumulation of HSPCs in the spleen caused by tumor-induced EMH, a detailed analysis of cell composition revealed that EPCs are abundant aggregating cells in the spleen. This phenomenon is mainly caused by the obstructed differentiation of EPCs and is also found in other EMH organs, such as the liver13. CD45 has been reported to be a marker of early erythroid precursors that can distinguish EPCs at relatively early and late differentiation stages14. Several studies have reported the accumulation of both CD45− and CD45+ EPCs in the spleen; however, CD45− and CD45+ EPCs have distinct mechanisms by which the EPCs are generated, as well distinct functional characteristics. Tumor-derived TGF-β contributes to the generation of splenic CD45− EPCs, which promote tumor progression via the neurotrophic factor, artemin (ARTN). Increased expression of ARTN and its receptor is correlated with a poor prognosis in patients with different types of cancer15. Recombinant EPO promotes resistance to radiotherapy and anti-PD-L1 therapy by restoring the levels of CD45− EPC and ARTN. Conversely, ARTN blockade augments the anti-tumor effects of anti-PD-L1 therapies in mice15,16.
Unlike CD45− EPCs, CD45+ EPCs have been shown to produce large amounts of reactive oxygen species (ROS) that inhibit systemic anti-tumor immunity of CD8+ T cells, ultimately increasing the susceptibility of bacteria and viruses and weakening anti-tumor immunity13. More importantly, scRNA-seq data have revealed that tumor-derived CD45+ EPCs lose key transcription factors for erythroid differentiation, such as Gata1, Klf1, and Sox6, and gained transcription factors for myeloid differentiation (i.e., PU.1), suggesting that CD45+ EPCs may have myeloid differentiation potential. Further research has revealed that GM-CSF produced by tumor cells mediates CD45+ EPCs trans-differentiation into the myeloid lineage and generates an erythroid-myeloid hybrid cell population, the erythroid-differentiated myeloid cells (EDMCs)17. Functional studies have shown that terminal differentiation of CD45+ EPCs into EDMCs achieves a wider variety of robust inhibition of T cell activation and attenuates the efficacy of anti-PD-L1 treatment by upregulating PD-L1, ICOS-L, NOX2, and Arg1 and generating high levels of TGF-β and IL-10. Clinically, high infiltration of intratumoral EDMCs is consistent with T cell exhaustion and an immunosuppressive microenvironment. Additionally, high infiltration of EDMCs is negatively correlated with the therapeutic efficacy of PD-1/PD-L1 antibodies in patients with cancer.
Another clinical study reported that CD45+ CD71+ erythroid cells accumulate in the peripheral circulation and tumor tissues of patients with hepatocellular carcinoma (HCC)18. These cells exhibit stronger suppressive functions by producing ROS, IL-10, and TGF-β, and the degree of infiltration can be used to predict the disease-free survival and overall survival of patients with HCC. Researchers have concluded that the number of CD45+ CD71+ erythroid cells in tumor tissues may be a reliable biomarker for HCC recurrence after radical surgery.
Tumor-associated anemia as a marker for EMH effectively predicts efficacy of PD-1/PD-L1 blockade
Tumor-associated anemia is the most common complication in > 30% of patients with cancer who are diagnosed prior to the initiation of antineoplastic therapy, especially in patients with advanced tumors. Between 40% and 70% of patients who are not anemic at the time of diagnosis also develop anemia after cancer treatment, which has a significant impact on survival, disease progression, treatment, and quality of life19,20. With respect to the mechanism by which tumor-associated anemia leads to a poor prognosis, it is currently thought that anemia aggravates hypoxia and promotes the Warburg effect, thus impairing antitumor immunity and reducing the efficacy of chemotherapy and radiotherapy. With the discovery that anemia triggers EMH not only by failing to replenish red blood cells, but also in generating immunosuppressive cells, such as splenic MDSCs and erythroid-derived immunosuppressive cells, anemia is widely recognized for its ability to inhibit antitumor immunity.
Recent analyses of cohorts of patients with various types of solid tumors who received PD-1/PD-L1 blockade showed that anemia is strongly associated with poor prognosis in most types of cancers17. Compared to patients with mild or normal hemoglobin (Hb) levels, patients with moderate-to-severe anemia (Hb < 90 g/L) have significantly shorter progression-free and overall survival. Moreover, no partial responses have been observed in patients with anemia and the best outcome is stable disease. As mentioned above, the deletion or inhibition of EMH-induced immunosuppressive cells significantly boosts the PD-L1 blockade effect11. Hence, the severity of tumor-associated anemia may serve as a potential predictor for the prognosis of patients with cancer and the therapeutic effect of immune checkpoint inhibitors, especially PD-1/PD-L1 blockade.
Summary
Tumor-induced signals and changes in the tissue microenvironment initiate tumor-induced EMH, which produces multiple populations of immunosuppressive cells, not only by promoting myelopoiesis of EMH-induced splenic HSPCs11,12, but also by accumulating EPCs in the spleen and mediating CD45+ subset differentiation into EDMCs13,15–17. These EMH-related immunosuppressive cells induce anti-tumor immune damage and immunotherapeutic resistance by impairing the proliferation and immune functions of CD8+ T cells. The deletion or inhibition of these cells reverse immunosuppression and improve the efficacy of immunotherapy. As a critical factor in EMH initiation and acceleration, anemia is also strongly correlated with the immunosuppressive status of patients and could be a valuable predictor of prognosis and immunotherapy efficacy.
Discussion
Researchers on the mechanisms of tumor-associated EMH and its effects on anti-tumor immunity have enhanced our knowledge of the hematopoietic system in tumor conditions; however, the underlying mechanisms that induce tumor-associated EMH and mediate EMH-associated immunosuppressive cell generation remain largely unclear.
Many clinical trials have reported that anemia is one of the most common complications of malignancies, for which there are numerous clinical management strategies. The usual management of anemia focuses on stimulating the proliferation and differentiation of progenitor cells, especially EPCs, by stimulators, such as EPO or EPO analogs, and supplying nutrition needed because anemia is mainly caused by dysfunction of bone marrow hematopoiesis and upregulated hemolysis. In the case of tumor-associated anemia, EPO stimulates erythropoiesis while the differentiation of EPCs is still obstructed, which can lead to more severe accumulation of EPCs and splenomegaly, followed by further EMH, immunosuppression, and resistance to the PD-L1 blockade15. In this case, remitting EMH and simultaneously correcting the differentiation behavior of HSPCs and EPCs which have accumulated in the EMH organs are more reasonable approaches.
Furthermore, current strategies to improve the efficacy of immunotherapy mainly involve the intervention of immunosuppressive cells, such as MDSCs and regulatory T cells. The effects of the current strategies are somewhat limited and conserved, suggesting that there are unknown cells that establish immunosuppressive functions to disrupt immunotherapeutic efficacy. Recent studies have revealed the existence and function of special immunosuppressive cells induced by tumor-associated EMH, as well as the possibility of reversing EMH progression and suppression. These findings provide new prospects for relieving resistance to anti-tumor immunity and improving immunotherapeutic efficacy. Therefore, apart from familiar immunosuppressive cells, attention should be paid to tumor-associated EMH, which promotes myelopoiesis and erythropoiesis. In conclusion, strategies for reversing tumor-associated EMH and targeting immunosuppressive cells generated by tumor-associated EMH should be developed based on the generation mechanisms and molecular signatures of these cells.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed the analysis: Bo Zhu and Haixia Long.
Wrote the manuscript: Zefang Chen, Xinyu Cheng, Li Yang and Xiaoming Cheng.
- Received June 6, 2023.
- Accepted July 3, 2023.
- Copyright: © 2023, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.