Abstract
Esophageal and gastric cancer (GC) are related to obesity and bariatric surgery. Risk factors, such as gastroesophageal reflux and Helicobacter pylori, must be investigated and treated in obese population. After surgery, GC reports are anecdotal and treatment is not standardized. This review aims to discuss GC related to obesity before and after bariatric surgery.
keywords
Introduction
Obesity remains an epidemic disease worldwide despite advanced clinical and surgical treatment over the past decades. Morbid obesity increases the risk of several diseases1, including cancer, and can lead to increased morbidity related to cancer treatment2,3.
The relationship between cancer and obesity is known, but whether high body mass index (BMI) significantly affects overall cancer risk or whether intense weight loss postoperatively influences cancer incidence remains unclear. A recent meta-analysis demonstrated reduced cancer risk after bariatric surgery, but conclusion could not be drawn because of the high heterogeneity between studies4. New research programs have focused on investigating proteins related to carcinogenesis that are hyper-expressed in obese patients and disappear after surgical weight loss.
Evidence of obesity as a risk factor for colon, breast, endometrial, and renal cell cancer is increasing5, but different results are obtained related to gastric cancer (GC). A recent meta-analysis by Turati et al.6 reviewed 22 studies and found a relative risk (RR) of 2.34 for BMI ≥30 kg/m2 for cardia and esophageal adenocarcinoma. The European Prospective Investigation into Cancer and Nutrition conducted a multicenter study that included 23 centers and almost 400,000 patients with 11-year follow-up; this study showed the correlation of the abdominal waist circumference and visceral fat with greater risk to esophagus-gastric junction (EGJ) cancer7.
In this context, we aim to review and discuss obesity and bariatric surgery and its risk factors related to GC.
Obesity as a risk factor for GC
Incidence of GC has decreased over the last decades. However, GC remains the fourth most common cancer worldwide and is usually in an advanced disease when it is diagnosed. This disease is classified into cardia and noncardia cancer, with distinct epidemiological and clinical characteristics and diverse treatment options8.
Even though the relationship between obesity and GC remains controversial, with a recent meta-analysis showing no statistically significant association between obesity and non-cardia GC, data on cardia cancer are suggestive9. A recent prospective study, including more than half a million people within the Me-Can Project10, showed that high BMI is associated with an increased risk for esophageal adenocarcinoma, independently from other metabolic risk factors, such as blood pressure, glucose, cholesterol, and triglycerides.
Risk factors
Helicobacter pylori (H. pylori)
H. pylori is classified a type I carcinogen in humans and a well-established risk factor for GC11. Following the continuity of gastritis, intestinal metaplasia, and dysplasia, H. pylori infection can lead to GC. Few studies have investigated the relationship between obesity and H. pylori infection12,13, and the evidence of obesity as a risk for gastric dysplasia is still poor14. Kim et al.15 studied more than 1,000 Korean patients to prove the association between obesity and GC regardless of H. pylori status. Their result showed an increased risk of early gastric cancer in men and gastric dysplasia in women, regardless of H. pylori infection.
Gastroesophageal reflux disease (GERD)
GERD and obesity are frequently related, and the prevalence of symptoms is correlated with high BMI16. Super-obese patients with BMI ≥50 kg/m2 exhibit higher prevalence of esophagitis compared with obese patients with BMI <40 kg/m2 17.
Barrett's esophagus, a premalignant precursor, is also highly associated with obesity; however, given the low incidence of disease progression, obesity markers and predictors have been investigated18,19. Higher level of ghrelin is associated with an increased risk of Barrett's esophagus20. Duggan et al.21 proved that increased levels of leptin and insulin resistance in overweight patients are associated with high risk of progression from Barrett's esophagus to EGJ cancer.
The Barrett's and Esophageal Adenocarcinoma Consortium22 study showed a positive association between heartburn/regurgitation symptoms and EGJ cancer. With regard to other risk factors, such as age, sex, and cigarette smoking, only BMI showed a different effect on stratified analysis with an increased risk for the obese group.
Inflammatory Factors
Obesity is associated with a low-grade chronic inflammation of white adipose tissue (WAT) that is related to insulin resistance and cardiovascular morbidity23,24. In obese patients, WAT activates the innate immune system and induces secretion of TNF-α and interleukin-625. In vivo and in vitro studies have shown that these cytokines are related to GC, inducing proliferation and inhibiting apoptosis of human GC cell lines26.
Pathological findings
Several surgeons from different countries with high rates of GC, such as Japan, Korea, and Chile, suggested Roux-en-Y gastric bypass (RYGB) with resection of the distal stomach because accessing excluded stomach postoperatively is difficult27,28. Braghetto et al.29 performed laparoscopic resectional gastric bypass in 112 obese patients, with 10.7% complication rate and only 2 duodenal stump leakages. Histological study found no GC and gastric dysplasia in only five patients.
Over the last 10 years, sleeve gastrectomy (SG) has gained popularity and provided an opportunity to examine the resected specimen, but no incidental finding of GC was reported. Several studies found high rates of gastritis and H. pylori infection on the pathological study of the resected stomach30,31; however, only case reports have described incidental findings of gastrointestinal stromal tumors (GIST)32,33. Given the scarce incidence of pathological findings, selective microscopic examination is endorsed as a safe and economic option, guided with clinical history and macroscopic examination34.
Pre-operative screening
Current guidelines recommend routine upper gastrointestinal endoscopy (UGE) before bariatric surgery35. As shown in Table 136–48, several studies identified a wide range of findings, such as gastritis, esophagitis, hiatal hernia, and gastric or duodenal ulcers, but no study found incidental GC36–38. Routine UGE has been questioned because most of the findings are clinically irrelevant.
Studies addressing routine upper gastrointestinal endoscopy before bariatric surgery
Incidental findings without clinical suspicion can lead to unnecessary and morbid treatments, such as prophylactic gastrectomy for gastric polyposis that revealed as benign lesion42. Fernandes et al.48 were the first to address endoscopic findings and surgical complications. In regression analysis, endoscopic ulceration was the only predictor of postoperative complications.
GC after bariatric surgery
Reported cases of GC after bariatric surgery are anecdotal, with low level of evidence, arising between 1 and 10 years postoperatively. A recent meta-analysis showed 18 cases of GC after different bariatric procedures, with a mean diagnosis at 8.6 years and adenocarcinoma representing 83% of the cases (Table 2)49–65.
Data reported on gastric cancer after bariatric surgery
Pathophysiological mechanism
Using the evidence of gastric stump cancer after Billroth II gastrectomy for peptic ulcer, anatomic changes after bariatric surgery may increase carcinogenic risk66. However, according to tumor location, a different pathophysiological mechanism can be established.
RYGB is still the most common bariatric procedure and has the risk of cancer in the gastric pouch and the excluded stomach. Despite increasing evidence showing that RYGB has a positive role on GERD, with clinical and endoscopic improvement67–69, recent studies have proven the presence of bile reflux in the pouch; its presence can be related to cancer risk as alkaline reflux is widely accepted as a cause of esophagitis, Barrett's esophagus, and adenocarcinoma70–72.
The excluded stomach has no contact with food and its exogenous carcinogens; thus, pancreaticobiliary reflux may be responsible for cancer risk. Bile reflux in the excluded stomach is well known, and its attributed mucosal changes with atrophy, intestinal metaplasia, and chronic inflammation have been tested in experimental models73. Endoscopic evaluation and cholescintigraphic study found bile in the excluded stomach in 68.6% and 36% of patients, respectively74,75.
For restrictive procedures, such as gastric banding and SG, the main mechanisms are GERD and its consequent mucosal transformations, food stasis causing chronic inflammation, and local irritation by the band. Previous studies showed that foreign body can lead to local pathological changes76, but evidence also exists that the pressure applied by the gastric band can cause ischemia and metaplastic changes in the mucosa that can progress to malignancy77.
Diagnostic assessment
Given the lack of surveillance program after bariatric surgery, a delay in diagnosis caused by unspecific symptoms is common. The most frequent complaints are dysphagia, nausea, vomiting, abdominal pain, and anemia, which are often attributed by physicians and patients to the effects of the bariatric surgery itself instead of a new disease78.
UGE is the first and most important test if a patient is suspected of GC. This test must be carried out by experienced hands and with great criteria, as well as with photographic documentation and biopsy of any suspected area. If any strictures are observed, obtaining complete dilation is significant to perform complete evaluation of the stomach.
Computed tomography (CT) scan can help to locally access tumor extension and distant metastasis. In the case of RYGB, CT can be used as an initial diagnostic tool because excluded stomach is difficult to evaluate. It has an important role in evaluating gastric distention and greater tumors, but early lesions can be misdiagnosed and biopsy cannot be performed56.
Several authors have reported different and innovative techniques to access the excluded stomach. Virtual gastroscopy was described in 200279 and has been performed through percutaneous and noninvasive techniques80. Despite the better image quality of virtual gastroscopy, it has the same limitations to small lesions and histologic evaluation as the CT scan.
Endoscopic evaluation of the excluded stomach is described both via retrograde approach and double-balloon technique. Retrograde endoscopy has long been reported, but failures are frequent due to the angulations of the anastomosis81. Double-balloon endoscopy was first described by Yamamoto et al.82 in 2001. This technique can access the excluded stomach even with long limbs and shows high success rate (87.5%), as reported by Safatle-Ribeiro et al.83.
Surgical treatment
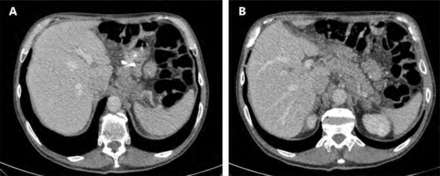
Given the unspecific symptoms and difficult diagnostic evaluation, several patients may present with locally advanced (Figure 1) or metastatic disease55. For these patients, multidisciplinary palliative treatment must be provided by surgeons, oncologists, radiotherapists, and nutritionists.
Computed tomography (CT) showing locally advanced gastric pouch cancer. (A) Pouch with silastic ring (B) Remanent stomach.
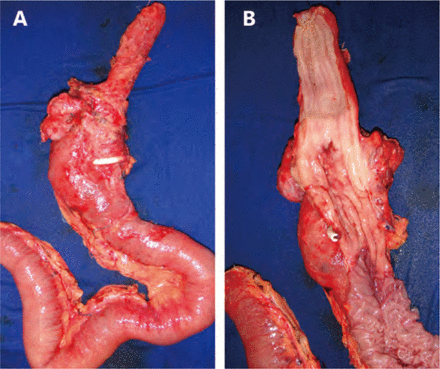
In cases amenable to surgical treatment, extent of resection is determined by tumor location84. Tumors of the pouch can be treated similarly to EGJ tumors by removing the gastric pouch, esophagus, and regional lymph nodes (Figure 2). In the case of a previous RYGB, the excluded stomach can be used for reconstruction and reach the neck for cervical anastomosis. Care must be taken to preserve the vascular supply of the greater curvature, particularly the right gastric and gastroepiploic arteries. If the bariatric procedure was a SG and the tumor is close to the cardia, a complete resection must be conducted and colon or jejunum can be used for reconstruction.
Surgical resection of gastric pouch cancer with subtotal esophagectomy. (A) Oesophagus, gastric pouch and alimentary limb (B) Detail of tumor in gastric pouch.
Neoplasms located in the excluded stomach are treated with total gastrectomy and D2 lymphadenectomy, following the same criteria for margins and extent of resection as GC treatment in general population. Nevertheless, resection of the Roux limb is unnecessary to preserve intestinal length and prevent nutritional complications.
Conclusions
Obesity is a risk factor for several types of cancer, including GC and EGJ. Reports of GC in gastric pouch or excluded stomach are rare, frequently asymptomatic and with incidental diagnosis. During bariatric surgery follow-up, late changes of symptoms, notably dysphagia, should be a warning sign for cancer diagnosis. Cancer treatment is ruled by current concepts, including surgical resection with D2 lymphadenectomy according to location with either gastrectomy of the excluded stomach or esophagectomy and pouch resection.
Footnotes
Conflict of interest statement No potential conflicts of interest are disclosed.
- Received January 22, 2016.
- Accepted February 16, 2016.
- Copyright: © 2016, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.
- 38.↵
- 39.
- 40.
- 41.
- 42.↵
- 43.
- 44.
- 45.
- 46.
- 47.
- 48.↵
- 49.↵
- 50.
- 51.
- 52.
- 53.
- 54.
- 55.↵
- 56.↵
- 57.
- 58.
- 59.
- 60.
- 61.
- 62.
- 63.
- 64.
- 65.↵
- 66.↵
- 67.↵
- 68.
- 69.↵
- 70.↵
- 71.
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵