Abstract
Adiponectin (APN), an adipokine produced by adipocytes, has been shown to have a critical role in the pathogenesis of obesity-associated malignancies. Through its receptor interactions, APN may exert its anti-carcinogenic effects including regulating cell survival, apoptosis and metastasis via a plethora of signalling pathways. Despite the strong evidence supporting this notion, some work may indicate otherwise. Our review addresses all controversies critically. On the whole, hypoadiponectinaemia is associated with increased risk of several malignancies and poor prognosis. In addition, various genetic polymorphisms may predispose individuals to increased risk of obesity-associated malignancies. We also provide an updated summary on therapeutic interventions to increase APN levels that are of key interest in this field. To date efforts to manipulate APN levels have been promising, but much work remains to be done.
keywords
Introduction
Obesity is defined as a chronic and excessive growth of adipose tissue. It is a growing health problem worldwide and has been described as a "global pandemic". Thus, obesity-associated diseases provide a substantial public health challenge, as they are a major cause of avoidable mortality and morbidity. In particular, excess adiposity is thought to be associated with about 20% of all cancers1.
Adipose tissue, originally thought as a passive depot for fat metabolism, is being increasingly recognized as an active endocrine organ. It secretes a wide array of bioactive molecules called adipocytokines, which act as key mediators in several obesity-associated diseases. Amongst these adipocytokines, adiponectin (APN), also known as the "guardian angel cytokine", has been proposed as having a key role in the pathogenesis of obesity-associated cancers along with other diseases such as cardiovascular disease2 and type 2 diabetes3,4. It is important to stress that adipocytes also produce many pro-inflammatory adipocytokines that have been implicated in the pathogenesis of cancer. The production harmony of these countering adipocytokines may represent the beauty of nature regulating oneself. Dysregulation of this harmony may signify the early development of diseases such as carcinogenesis.
Tempering this axis of disharmony may represent an opportunity to correct disease process. Hence, the possibility of targeting APN and its signalling pathways therapeutically has led to a surge in interest in this field and several recent developments, which we are reviewing here.
APN structure
APN is produced mainly from white adipose tissue, but also in lower quantities from brown adipose tissue5. Reports are also found suggesting that APN is expressed, but at much lower concentrations, in several other tissues such as skeletal muscle6, cardiomyocytes7, liver8, bone marrow9 and cerebrospinal fluid10.
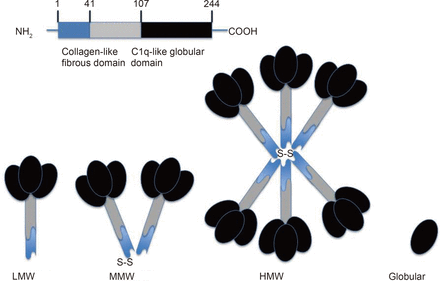
Monomeric APN is a 30kDa gylcoprotein composed of 244 amino acids11. APN is encoded on human chromosome 3q27 by the ADIPOQ gene12. Structurally, APN consists of a signal peptide domain at the N-terminus, a short variable region, a collagenous domain and a C-terminal globular domain, which is homologous to C1q13. Thus, with its C-terminal domain, APN structurally belongs to the C1q/tumor necrosis factor (TNF) superfamily14.
A globular version of APN (gAcrp) also exists at small concentrations in plasma. It comprises of the C-terminal globular domain formed from proteolytic cleavage. Full-length APN (flAcrp) can exist in a variety of different isoforms (Figure 1), with monomeric APN able to trimerize to form low molecular weight (LMW) APN. Two trimers can then self-associate to form a middle molecular weight (MMW) hexamers. The trimers are also able to form 12- or 18-mers [high molecular weight (HMW) APN] via disulphide bonds. Post-translational modifications are thought to be critical to oligomer formation, particularly HMW APN. These post-translational modifications are also important for APN's receptor binding and biological activity. Monomeric APN is thought to be found only in adipocytes, whereas oligomeric APN is present in the circulation at concentrations of around 5-30 μg/mL. Active HMW or flAcrp appears to be present at higher concentrations, but LMW and gAcrp are also present in the circulation at low levels, possibly because of a shorter half-life. The HMW isoform is the most biologically active. The different isoforms of APN may also mediate different effects in different tissues. For example, the HMW isoform has been suggested to mediate the pro-inflammatory actions of APN, whereas the LMW isoform may be responsible for its anti-inflammatory activity15.
APN's structure. This figure depicts APN's structure molecularly and schematically.
However, APN concentration levels are thought to be altered in various disease states, with them being reduced in type 2 diabetes16, coronary heart disease and atherosclerosis17,as well as obesity and insulin resistance18. In addition, APN levels have been seen to be reduced in several cancers.
APN receptors
To date, three APN receptors have been discovered. These are AdipoR116, AdipoR216 and the more recently found T-Cadherin19.
The two classical APN receptors, AdipoR1 and AdipoR2, are seven transmembrane domain receptors with an internal N-terminal region and an external C-terminal region. This structure is the opposite of that seen in other G-protein coupled receptors16. These two receptors have highly comparable structures with their protein sequences sharing 67% homology20. AdipoR1 is a 42.4kDa protein, whereas AdipoR2 is a 35.4kDa protein. Recent study of the crystal structures of these two receptors shows that they have a large cavity, where three histidine residues co-ordinate a zinc ion21. This is seen to be crucial to APN receptor interactions such as 5' adenosine monophosphate-actiavted protein kinase (AMPK) phosphorylation and uncoupling protein 2(UCP2) upregulation21.
Studies utilizing small-interfering RNA (siRNA) show that AdpoR1 has a high affinity for gAcrp and a low affinity for flAcrp, whereas AdipoR2 has moderate affinity for both gAcrp and flAcrp16. AdipoR1 is ubiquitously expressed and is particularly abundant in skeletal muscle and endothelial cells, whereas AdipoR2 is highly present in the liver22. AdipoR1 and AdipoR2 may form both homo- and heteromultimers. Both of these receptors have been detected in almost every normal and malignant tissue, but one receptor usually prevails in each tissue. In obese individuals, AdipoR1 and AdipoR2 expression seems to be reduced23, which thus leads to decreased sensitivity to APN.
In addition, the third non-classical receptor for APN is T-cadherin, which is a glycosylphospatidylinositol-anchored protein that lacks a transmembrane domain. This cell-surface receptor is found in endothelial, epithelial and smooth muscle cells. T-cadherin is encoded on the cadherin-13 gene and can bind MMW and HMW APN, but not trimeric or globular APN24. Calcium dependent mechanisms are seen to be crucial to T-cadherin signalling24.
Genetic mutation of all receptors has been noted. For example, no missense or nonsense mutations in AdipoR1/R2 were detected in patients with severe insulin resistance. It was shown that none of the 24 polymorphisms (allele frequency of 2.3%-48.3%) tested was associated with the type 2 diabetes. It was concluded that genetic variation in AdipoR1/R2 is not a major cause of insulin resistance in humans, nor does it contribute in a significant manner to type 2 diabetes risks25. Of course, this is only a small study, and therefore further exploration on genetic mutation of APN or its receptor may offer better understanding on the interplay of disease and genetics.
APN signalling
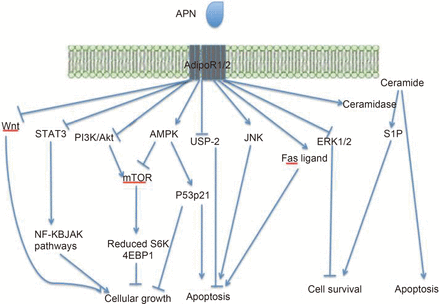
APN binds to its receptors and subsequently modulates a plethora of signalling pathways, exerting a variety of complex metabolic and immunological effects. These effects are mostly mediated via AMPK17,26–28, mitogen activating protein kinase (MAPK)28,29, phospho inositide 3-Kinase (PI3K)/Akt27,28, mammalian target of rapamycin (mTOR)30, c-Jun N-terminal kinase (cJNK)31,32, signal transducer and activator of transcription 3 (STAT-3), sphingolipids33, Wnt34, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)28 signalling. These anti-cancer signalling pathways are summarized in Figure 2.
A summary of the cancer protective signalling pathways modulated by APN in vivo. APN may activate or inhibit these pathways either when it is presented directly or indirectly to AdipoR1/2. APN activates AMPK, Fas ligand and JNK, whereas it inhibits Wnt, STAT3, PI3K/Akt, USP-2, and ERK1/2. APN also promotes ceramidase activity, increasing the conversion of ceramide to S1P. Arrowheads: activating pathways; Blunt ends: suppressive pathways.
A large proportion of APN's effects are exerted via the AMPK pathway26,27. A tumor microenvironment often exhibits certain features such as hypoxia, redox imbalance and nutrient starvation35–37. These features can lead to an increase in the AMP/ATP ratio and hence AMPK activation38,39. This has several pleiotropic metabolic effects, which generally restore cellular energy. AMPK also disrupts cellular growth signalling via mTOR and thus exerts its anti-cancer effects40,41. This inhibition of the mTOR pathway decreases translation via S6 kinase (S6K) and the eukaryotic translation initiation factor 4E binding protein-1 4EBP1 phosphorylation pathways via tuberous sclerosis 2 (TSC2) phosphorylation30. AMPK is also able to promote growth arrest and apoptosis via enhanced p53 and p21 expression42.
APN also exerts its effects via the PI3K/Akt axis26. Activation of PI3K leads to a cascade of events resulting in cellular survival, growth and proliferation43,44, and an increase in glycolysis and fatty acid synthesis45. APN has been seen to have direct46 and indirect47 inhibitory effects on the PI3K/Akt pathway. Akt also has opposing effects to AMPK on TSC2, inhibiting its action37,48,49.
Moreover, APN influences the MAPK cascade, which involves cJNK, MAPKp38 and extracellular signal-regulated kinases (ERK)1/2. cJNK and MAPKp38 are seen to have variable effects on cell proliferation and apoptosis, whereas ERK1/2 has largely mitogenic effects50. APN treatment has been seen to cause increased cJNK signalling and hence apoptosis via caspase 3 in a HC cell line51. Indeed, APN has also been shown to activate other caspases such as 8 and 9 in order to promote apoptosis52. APN has inhibitory effects on ERK1/2 signalling, which in BC53, LC54 and EC55 cells has been shown to lead to reduced cell viability. APN treatment in BC cells led to an increase in p53 and Bax expression and a decrease in c-myc, cyclin D and Bcl-2 (B-cell CLL/Lymphoma 2) expression, which subsequently led to cell cycle arrest and apoptosis56.
STAT3 has several cancer-causing effects including increasing tumor cell proliferation, survival, angiogenesis and invasion, as well as inhibiting anti-tumor immunity57. STAT3 can stimulate pro-oncogenic inflammatory pathways such as NF-κB and interleukin (IL)-6-Janus tyrosine kinase (GP130 JAK) pathways, and inhibit anti-tumor pathways such as STAT1- and NF-κB-mediated T-helper cell (Th1) pathways57. As well as its direct effects as a transcription factor, STAT3 is seen to have epigenetic effects on gene expression and also modulates mitochondrial functions58. gAcrp and flArcp have both been seen to inhibit STAT3 activation in PrC and HC lines59,60.
APN is also thought to have inhibitory effects on Wnt signalling34. Wnt binds to its receptor, frizzled, in order to inactivate glycogen synthase-3β (GSK3β) and to enhance nuclear accumulation of β-catenin34. Wnt signalling has positive effects on cellular growth and proliferation. APN has been shown to stimulate Wnt-inhibitory factor 1, which antagonises Wnt signalling and cancer progression61. APN has also been shown to modulate Wnt signalling in MDA-MB-231 BC cells62.
Furthermore, APN was seen to decrease ubiquitin specific protease 2 (USP-2) expression in HC and BC cells63 and thus this may be important in APN's anti-proliferative effects via cyclin D1 degradation64. USP-2 has also been seen to prevent apoptosis65 and cause malignant transformation, via p53 regulation66, in prostate cancer cells.
As well as decreasing cyclin D1 activity, gAcrp has been shown to increase p27 activity in HC (HepG2) and BC [Michigan Cancer Foundation-7 (MCF-7)] cells67. p27 has been considered as a tumor suppressor gene, with p27 dysfunction causing excess cell cycle activity and carcinogenosis68. In these HepG2 and MCF-7 cell lines, gAcrp induced Fas ligand activity and hence promoted apoptosis67.
However, it must be remembered that APN has also been shown to have proliferative effects on cancer cells69. For example, APN binding to AdipoR1/R2 can also promote ceramidase activity, which subsequently acts to enhance ceramide catabolism to the proliferation enhancer and anti-apoptotic metabolite sphingosine-1-phosphate (S1P)33. In general, it is thought that higher APN concentrations may have a proliferative effect and lead to tumor growth, whereas lower concentrations are generally thought to have anti-carcinogenic effects. Furthermore, the presence of various isoforms of APN further complicates the picture and is a reason for the pleiotropic effects seen.
APN in cancer
Obesity is seen as a risk factor for several cancers and thus, it is thought that adipokines such as APN may act as crucial mediators in the pathogenesis of obesity-associated malignancies. Generally, serum APN levels are seen to be reduced in cancer. Here we will look at several examples of cancers where APN levels are seen to confer altered risk and prognosis for the patients affected.
Serial measurements of adipokines during cancer diagnosis and its treatments have elegantly demonstrated70. In children with acute lymphoblastic leukemia (ALL), low serum APN, and high serum leptin and resistin level were present at diagnosis70. Adipocytokines alterations are progressively restored during therapy, representing the success of treatment and good health. One should not undermine the complexity of disease and its treatment, these observations merely indicate the association of adipocytokines, cancer and cancer therapy.
Breast cancer (BC)
Miyoshi et al.71 first highlighted the relationship between hypoadiponectinaemia and increased BC risk, where BC patients with lower APN levels being more likely to show a more aggressive phenotype. A recent meta-analysis of 8 observational studies found that low APN levels are associated with increased risk of BC in post-, but not pre-menopausal women72. The risk reduction from higher APN levels has been reported to be around 65%-80% in early BC patients73.
However, some studies have produced inconsistent and confusing results, with one study showing no association between APN levels and BC risk74. This may in part be explained by the presence of different isoforms of APN. In particular, low levels of the HMW isoform may be predictive of increased BC risk, especially in post-menopausal women75.
Although the mechanisms by which APN exerts its protective effects in BC are largely unknown, it has been proposed that one mechanism may involve APN altering the sensitivity of peripheral tissue to insulin. Insulin levels are generally increased when APN levels are lower and hence these increased insulin levels may induce BC cell proliferation via insulin and insulin-like growth factor 1 (IGF-1) receptor function72.
Further work went on to establish that BC treatments might alter the balance of adipokines to favour anti-cancer state. A decreased leptin to APN ratio (LAR) were found in hormonal therapy groups76. These changes might have occurred through both mechanisms of hormonal therapy and body composition changes. It has been suggested that hormonal treatment may exert their protective effects for BC patients by decreasing LAR.
Our own work has also indicated that a normal serum APN was noted in the stage I and II BC, however, its level decreased with the progression of stages, to a level that was statistically significantly28. This work indicates that the disharmony of adipokines become very obvious when the disease advancing despite its treatments (either with chemotherapy or hormonal treatment). The treatment may try to restore the normal harmony of adipocytokine production, but ultimately the final stage of disease shapes the landscape of these adipocytokines. Therefore, many have argued that the serial measurement of adipocytokines maybe a good surrogate marker for the disease progression.
APN may also regulate the expression levels of various molecules such as MAPK, Bax, p53, Bcl2, AMPK, p42/p44 MAPK, cyclin D1 and β-catenin72. APN can also reduce the proliferation of BC cells independent of estrogen receptor status62.
Furthermore, single nucleotide polymorphisms (SNPs) for ADIPOQ (rs2241766 and rs1501299) and ADIPOR1 (rs7539542) were significantly associated with BC risk77,78. The importance of this association may also be influenced by ethnicity78. However, one study did not find any significant association between ADIPOQ, ADIPOR1 and ADIPOR2 SNPs and BC risk79.
Colorectal cancer (CRC)
Obesity, insulin resistance and hyperinsulinaemia have all been associated with CRC pathophysiology. A negative correlation between plasma APN levels and CRC risk has been shown by several studies and meta-analyses80–83. It has been suggested that APN interactions with AdipoR1 are more important to its protective effects than interactions with AdipoR2 as seen using AdipoR1 and 2 knockout mice models84. This group also found these protective effects to only be apparent under a high-fat but not basal diet. AdipoR1/R2 expression levels may also be increased in CRC, due to reduced APN levels85.
APN interactions may be crucial at initial and later stages of CRC. It was seen that as APN levels decreased, the number and size of tumors increased86. Furthermore, APN receptor levels were seen to be significantly lower in CRC compared to adenomatous polyps87. AdipoR2 has also been positively linked with tumor, node and metastasis staging in CRC88.
In vitro studies show that APN may directly affect cell proliferation, adhesion, invasion and colony formation as well as controlling metabolic (via AMPK/S6 signalling), inflammatory [via STAT3/VEGF (vascular endothelial growth factor)] and cell cycle (via p21/p27/p53/cyclins) processes in CRC cell lines89.
It has also been suggested that certain gene polymorphisms may increase susceptibility of developing CRC. Variant rs1342387 of the ADIPOR1 gene and variants rs2241766 and rs1501299 may increase CRC risk at least in some populations90–92.
Endometrial cancer (EC)
A significant inverse relationship has been found between APN levels and EC risk93–97, as well as between the LAR ratio and EC risk97. In particular, one recent meta-analysis showed that higher APN levels were associated with a 53% reduction in EC risk93. This meta-analysis also identified this relationship to only be significant in post-menopausal women, whereas others argue that the relationship is stronger in pre-menopausal women94. Thus, clarification of these influences may be required in future studies.
APN may exert its protective effects on EC risk via several pathways and processes. One key mechanism may be via decreasing insulin levels98 and hence reducing carcinogenesis through estrogen99. APN's anti-inflammatory actions may also be particularly relevant to EC100. Reduced MMW APN was identified as the only isoform that was an independent risk factor for EC101, and thus may be particularly important for EC. However, the reason why this is the case is yet to be determined.
An increased frequency of ADIPOQ variant rs1063539C was significantly associated with decreased EC risk in a recent study102. Another study found that ADIPOQ variants rs3774262, rs1063539 and rs12629945 were correlated with EC risk although not to a significant level103. No such relationship for ADIPOR1 and ADIPOR2 genes were found103.
Gastric cancer (GC)
APN levels were seen to have a significant inverse correlation with GC risk104,105. GC cells lacking AdipoR1 had significantly higher lymphatic metastases and peritoneal dissemination compared to those that were AdipoR1 positive106. However, another study found no significant prognostic value in AdipoR1/2 expression in GC107.
A recent study has shown that ADIPOQ variant rs266729 may be an independent prognostic risk factor for never-drinking GC patients receiving surgical treatment108. In addition, ADIPOQ variant rs16861205 and ADIPOR2 variants rs10773989 and rs1044471 were significantly associated with decreased risk of cardia GC109. However, rs16861205 was also significantly associated with increased risk of body GC109.
Esophageal cancer (OC)
Low APN levels have been associated with increased risk of OC110–113. Low AdipoR1 expression was also an independent predictor for overall survival and AdipoR2 expression was inversely associated with tumor size114.
Pancreatic cancer (PC)
An inverse relationship between APN levels and PC risk has been shown115,116. However, a certain amount of heterogeneity exists in the literature, with reports of a positive correlation between APN levels and PC risk117–119. Thus, clarification of APN's role in the PC is needed as it may be that APN exerts unconventional roles in certain tissues. This elevation in APN concentrations seen in PC may also be a compensatory mechanism for weight loss and inflammation during cancer cachexia, and due to decreased expression of APN receptors118.
One study also reported that the ADIPOQ SNP rs1501299 may be associated with PC risk120.
Hepatic cancer (HC)
There appears to be significant heterogeneity in studies associating APN levels and HC risk. Higher APN levels have been associated with increased risk of primary HC in Japanese individuals with hepatitis121. These higher APN levels may also predict poorer survival in HC patients122,123. However, one study124 found APN and AdipoR1/R2 expression levels to be significantly lower in HC patients compared to controls, and lower AdipoR1/R2 expression was associated with poor prognosis in HC patients. This proposed protective role might at least in part be mediated via thioredoxin protein-dependent apoptosis125. Thus, the exact role of APN in HC remains to be determined.
The rs1501299 variant of ADIPOQ may also predispose an increased risk of developing HC risk126.
Renal cell carcinoma (RCC)
Serum APN levels have been negatively correlated with RCC, its tumor stage and its metastasis127–129. However, a more recent study found a positive association between APN levels and RCC risk, which appeared to vary with ethnicity130. This may highlight the complicated picture presented by different APN multimers in RCC. The rs182052 SNP for ADIPOQ has also recently been associated with RCC risk131 and lower APN levels.
Prostate cancer (PrC)
Lower APN levels have been associated with increased PrC risk132–134. Furthermore, reduced APN levels were associated with tumor stage in obese and overweight men, but not in normal weight men or overall in all men135. Other studies have also found no significant association between APN levels and PrC risk136,137. Interestingly, APN was also seen to have anti-proliferative, but not anti-apoptotic effects on human PrC cells138.
A study found four ADIPOQ SNPs (rs266729, rs182052, rs822391 and rs2082940) to be significantly associated with overall PrC risk139. However, this association may be influenced by ethnicity as it had a predominantly Caucasian population, whereas another study found no significant association between ADIPOQ and ADIPOR1 SNPs and PrC risk in African Americans140.
Lung Cancer (LC)
Many studies have found no significant association between APN levels and lung cancer (LC) risk141–143. However, one study did find an increase in APN levels in LC patients compared to controls144. In contrast, one study141 found APN levels to be reduced in advanced disease patients compared to limited disease patients. Thus, the exact role of APN in LC remains to be determined.
Possible protective APN signalling interactions may be mediated via AdipoR1, as a study found AdipoR1, but not AdipoR2, expression to be positively associated with increased survival145. Rs266730, an APN promoter polymorphism, was also associated with LC risk in a recent study146. Another study found ADIPOQ variant rs2241766 to be associated with risk of non-small cell LC and its prognosis after surgery147.
Haematological cancers
APN has been associated with various haematological cancers including leukemia, lymphoma and myeloma. APN levels were significantly lower in acute myeloid leukemia (AML) and ALL patients148. Hypoadiponectinaemia has also been associated with chronic myeloid leukemia (CML)149. Decreased levels of APN are also linked to an increased risk of myeloma150. APN levels were also lower in chronic lymphoid leukaemia (CLL) patients than controls151. In contrast, APN levels were higher in non-Hodgkin's lymphoma patients than controls152.
The relationship between APN levels with the risk and prognosis of various cancers is summarized in Table 1. The altered risk of various malignancies conferred by ADIPOQ, ADIPOR1 and ADIPOR2 gene polymorphisms is summarized in Table 2.
Summary of clinical data showing the association between APN and risk and prognosis of various cancers
Summary of the effects of gene polymorphisms on the risk of various cancers
APN and cancer metastasis
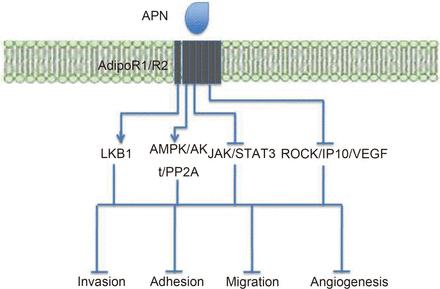
Metastasis is a complex and critical aspect of cancer from a clinical perspective, with it having been estimated to be the cause of around 90% of deaths from cancer153. However, despite its importance little is known about this process. APN has been seen to suppress many crucial metastatic processes such as adhesion, invasion and migration of BC cells154. This may occur in an liver kinase B1 (LKB1)-mediated manner155. LKB1 expression is seen to be inhibited in BC in situ cases associated with invasion, but not those without invasion and hence this pathway may be critical to metastasis154. APN's protective role against metastasis may also in part be mediated via the AMPK/Akt pathway156.
Furthermore, APN has been seen to negatively impact upon angiogenesis and invasion in liver tumor nude mice models157. In vitro studies from this group showed that APN reduced the expression of the Rho-associated protein kinase (ROCK)/interferon gamma-induced protein 10 (IP10)/VEGF signalling and suppressed lamellipodia formation, which is required for cell migration157.
APN was also seen to have metastatic suppressive effects in EC cells158. Here, APN was seen to inhibit leptin-mediated invasion, which required inactivation of JAK/STAT3 signalling and activation of the AMPK pathway. These anti-metastatic effects of APN are summarized in Figure 3.
Summary of APN's anti-metastatic effects and the signalling pathways involved. The LKB1 and AMPK pathways inhibit metastasis, whereas JAK/STAT3 and the ROCK/IP10/VEGF pathways promote metastasis. APN activates LKB1 and AMPK signalling, and inhibits JAK/STAT3 and ROCK/IP10/VEGF signalling. These effects lead to inhibition of invasion, adhesion, cell migration and angiogenesis, overall reducing the likelihood of metastasis.
How important is APN in malignancies?
Here, we present compelling evidence suggesting that APN may be a key molecule in the pathogenesis of several malignancies associated with obesity. However, it must be pointed out that this link is not a simple one, as the strength of this association seems to vary depending on factors such as tissue type, age, menopausal status, ethnicity and sex. In addition, it must be appreciated that several other important factors may also contribute to the link between obesity and malignancies. These include diet (calorie intake and specific components of the diet)159, physical activity160, altered insulin sensitivity161, the action of insulin like growth factors (IGFs)162, sex hormones163, the NF-κB system164, and the importance of genetics, oxidative stress and vascular growth factors independent of the action of APN. Thus, a goal of future studies will be to evaluate the relative importance of these mechanisms in cancer of different tissues and in different sub-groups.
In circumstances when the cancer is advanced, it often results in severe weight loss and cancer-related cachexia. Hyperadiponectinaemia may be noted as seen in cases with anorexia nervosa165. This transient and late increase in APN level has very little influence in the late phase of the pathogenesis of obesity-related cancers, as the disease modifying effects of APN has already altered the course of disease in the early phase. Moreover, it was recently reported that an increase in dendritic cell signalling of APN receptors, in particular AdipoR1/R2 following their engagement with APN can blunt the tumor-specific immune response in the patients with metastatic diseases28. This in fact results in a detrimental effect on ones' ability to control cancer28. Therefore the tempo of low or high APN level on the disease process can influence greatly the outcome of disease, depending on the stages of the cancer. The applicability of APN as a therapeutic tool to modify the disease outcome needs to take the stage of cancer into account when considering it.
Therapeutic potential
Thus, due to its importance in the carcinogenesis and progression of several cancers, APN has been seen as a promising therapeutic target. APN may act as a possible prophylactic as well as a therapeutic. Nevertheless, efforts to engineer the APN protein have been troublesome, partly due to a lack of clarity on the effects of different APN isoforms. The requirement of post-translational modifications also further complicates the picture and means that mammalian cells are required. Hence, it may be more lucrative to screen for existing agonists or enhance endogenous APN levels.
The first APN receptor agonist that was produced is called ADP355 and it binds to both AdipoR1/R2, but with a greater affinity for AdipoR1166. This protein includes several non-natural amino acids, which stabilize the structure and protect it from proteolysis. In vivo, ADP355 inhibits orthotopic human BC xenograft growth by 31%, with an acceptable safety profile. It was also seen to regulate several signalling pathways including AMPK, PIK3/Akt, STAT3 and ERK1/2 in a manner similar to gAcrp.
More recently, this group aimed to modify ADP355 in order to potentially optimize its protective effects167. They showed that a substitution of the Gly4 and Tyr7 residues with Pro and Hyp led to a 5-10 fold increased agonistic activity. In addition, they also developed a chimera from ADP355 and the leptin receptor antagonist, Allo-aca. It is hypothesized that this chimera may offer the potential for combination therapy in a single chemical entity and thus, allowing a much lower dose than a physical mixture of the two individual drugs.
After screening several molecules168, AdipoRon was seen to bind to AdipoR1/R2 at low micromolar concentrations. It was found to have similar effects to APN in muscle and liver, with downstream effects on AMPK and PPAR-α signalling168.
Using a high throughput assay, 9 naturally occurring compounds were discovered169. The most active AdipoR1 ligands were matairesinol, arctiin, (-)-arctigenin and gramine, whereas the most active AdipoR2 ligands were parthenolide, taxifoliol, deoxyschizandrin and syringin. These compounds were seen to share some of the effects of APN including anti-proliferative, anti-inflammatory and anti-cancer properties.
In addition, it is also possible to augment endogenous APN levels. PPARγ ligands have been suggested as a promising means of exploiting this mechanism, with a group showing that thiazolidinediones (synthetic PPARγ ligands) can increase APN levels in vitro and in vivo in a dose- and time-dependent manner170. These PPARγ agonists can be either full or selective and can either augment circulating APN levels or activate APN signalling via its receptor interactions. Selective PPARγ agonists are however thought to be safer and thus show greater promise.
One such selective PPARγ agonist is efatutazone, which although it showed promise in phase 1 trials on metastatic cancer patients171,172, failed to show sufficient efficacy in phase 2 trials and the study was thus terminated173,174.
Other PPARγ agonists that show promise include rivoglitazone and troglitazone175. Rivoglitazone has been shown to have beneficial effects on the insulin resistance, type 2 diabetes and atherosclerosis in vivo partly through its substantial effects on APN176,177. However, its effects on cancer remain to be studied.
Troglitazone and its synthetic derivative Δ2-troglitazone were shown to enhance APN gene and protein expression in a dose- and time-dependent manner. Troglitazone has been shown to have beneficial effects in vitro including preventing tumor cell invasion178. Δ2-troglitazone was seen to be more potent at reducing cell proliferation in cancer cells179 and may have a different side-effect profile to troglitazone180. However, similarly to efatutazone, phase 2 trials for troglitazone have been disappointing181,182 uncovering little clinical value in this PPARγ ligand. There have also been some concerns regarding the risk of harmful cardiovascular effects of thiazolidinediones, especially for rosiglitazone183. Known agonists of AdipoR1/R2 and potential therapeutic strategies are summarized in Table 3.
Summary of known agonists of AdipoR1/R2 and molecules that could potentially be exploited clinically
APN may also be modulated with dietary or lifestyle factors. For example, daily intake of fish or omega-3 supplements led to increased APN levels in the range of 14%-60%, whereas fiber supplementation led to an increase of 60%-115% in APN levels184. Moderately intense aerobic exercise has also been shown to elevate APN levels up to 260%185. Other dietary factors include coffee186, deep yellow vegetables187 and a Mediterranean diet188.
However, it must be remembered that modifying these receptor interactions and thus the metabolic effects of APN receptor binding can also have important and dire effects on anti-cancer immunity189. Thus, we believe this will be an important consideration in future developments in APN-based anti-cancer therapies. In addition, several potential side-effects from chronic APN therapy have been proposed. These include infertility, cardiac damage and reduced bone density190,191.
Conclusions
Our understanding of cancer including obesity-associated malignancies is rapidly improving. APN has come under recent scrutiny as a key mediator between obesity and cancer. Hypoadiponectinaemia is often found in several cancers and associated with poor prognosis. Hence, various efforts aiming to utilize the anti-cancer properties of APN therapeutically and prophylactically are being investigated. Furthermore, efforts to identify ADIPOQ, ADIPOR1 and ADIPOR2 SNPs that confer altered risk of cancer development may enable early screening and APN level augmentation.
We believe this field holds promise, but there remain several challenges to utilizing these treatments routinely. A deeper understanding of the cellular and molecular functions of APN in cancer is required in order for the development of effective therapies. The role of each isoform in distinct tissues and under tumor-specific conditions needs to be clarified. Furthermore, the molecular conditions under which APN acts as cancer suppressing or cancer promoting, anti-inflammatory or pro-inflammatory adipocytokine needs to be evaluated. The exact roles of each receptor and signalling pathway in different cancers also remain largely unknown. We believe these will be key steps in the pursuit of an effective APN-based cancer therapeutic. The complexity of APN influences the anti-tumor immune response and needs to be considered carefully when applying it as a therapeutic target.
Footnotes
Conflict of interest statement Peng H. Tan's research activities (with Oxford University) used to be funded by Sir Peter Morris's Surgeon Scientist Programme. Currently he works full-time for the NHS (UK) trust with an honorary role with UCL.
- Received October 23, 2015.
- Accepted January 5, 2016.
- Copyright: © 2016, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.
- 82.
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.
- 92.↵
- 93.↵
- 94.↵
- 95.
- 96.
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.
- 112.
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.
- 129.↵
- 130.↵
- 131.↵
- 132.↵
- 133.
- 134.↵
- 135.↵
- 136.↵
- 137.↵
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.
- 143.↵
- 144.↵
- 145.↵
- 146.↵
- 147.↵
- 148.↵
- 149.↵
- 150.↵
- 151.↵
- 152.↵
- 153.↵
- 154.↵
- 155.↵
- 156.↵
- 157.↵
- 158.↵
- 159.↵
- 160.↵
- 161.↵
- 162.↵
- 163.↵
- 164.↵
- 165.↵
- 166.↵
- 167.↵
- 168.↵
- 169.↵
- 170.↵
- 171.↵
- 172.↵
- 173.↵
- 174.↵
- 175.↵
- 176.↵
- 177.↵
- 178.↵
- 179.↵
- 180.↵
- 181.↵
- 182.↵
- 183.↵
- 184.↵
- 185.↵
- 186.↵
- 187.↵
- 188.↵
- 189.↵
- 190.↵
- 191.↵