Published online Feb 14, 2016. doi: 10.3748/wjg.v22.i6.2046
Peer-review started: June 25, 2015
First decision: July 10, 2015
Revised: September 18, 2015
Accepted: November 30, 2015
Article in press: November 30, 2015
Published online: February 14, 2016
Glucose metabolism in gastric cancer cells differs from that of normal epithelial cells. Upregulated aerobic glycolysis (Warburg effect) in gastric cancer meeting the demands of cell proliferation is associated with genetic mutations, epigenetic modification and proteomic alteration. Understanding the mechanisms of aerobic glycolysis may contribute to our knowledge of gastric carcinogenesis. Metabolomic studies offer novel, convenient and practical tools in the search for new biomarkers for early detection, diagnosis, prognosis, and chemosensitivity prediction of gastric cancer. Interfering with the process of glycolysis in cancer cells may provide a new and promising therapeutic strategy for gastric cancer. In this article, we present a brief review of recent studies of glucose metabolism in gastric cancer, with primary focus on the clinical applications of new biomarkers and their potential therapeutic role in gastric cancer.
Core tip: Increased glucose consumption is a hallmark of cancer cells. Studies focusing on glucose metabolism provide a new perspective on gastric carcinogenesis and a novel approach to exploration of biomarkers and therapeutic targets in gastric cancer.
- Citation: Yuan LW, Yamashita H, Seto Y. Glucose metabolism in gastric cancer: The cutting-edge. World J Gastroenterol 2016; 22(6): 2046-2059
- URL: https://www.wjgnet.com/1007-9327/full/v22/i6/2046.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i6.2046
Gastric cancer is one of the most common cancers worldwide and ranks second in cancer-related deaths[1,2]. Recent advances in cancer diagnosis and treatment have resulted in limited improvement in gastric cancer-related mortality[3]. Estimates even suggest that gastric cancer-related mortality will continue to increase[4]. To improve the survival rate, several studies have elucidated molecular mechanisms of gastric cancer, and identified biomarkers predicting prognosis and response to treatment[5,6]. A few biomarkers have been used as therapeutic targets for advanced gastric cancer[7]. However, the therapeutic results are still unsatisfactory, which may be due to multiple genetic variations and changes in microenvironment, such as altered glucose metabolism promoting gastric carcinogenesis.
Several decades ago, alteration of glucose metabolism in cancer cells, termed “Warburg effect”, was described. This discovery has revitalized the interest in the role of glucose metabolism in oncology since the widespread use of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) to evaluate various types of malignant tumors[8]. Compared with genomics and proteomics, metabolomics is a recent “omic” technique and the last step before phenotype, which provides new insight into pathophysiologic mechanisms in carcinogenesis. In fact, these biological pathways are not independent but co-dependent and co-operative in the progress of carcinogenesis.
The high mortality rate of gastric cancer is due to delayed diagnosis and lack of effective therapies for metastasis. Gastric cancer is generally screened using endoscopy and serum carbohydrate antigens, such as carcinoembryonic antigen (CEA). Clinical application of endoscopy is limited because of its relative invasiveness, cost and technical complexity, even in high-incidence countries of east Asia[9]. Additionally, the current serum biomarkers have poor sensitivity and specificity for gastric cancer. Therefore, new biomarkers that are non-invasive and enable stratification of patients with high sensitivity and specificity for screening, diagnosis, prognosis, prediction, and monitoring of aggressive and advanced gastric cancer are needed. Metabolomics facilitates the investigation of these biomarkers via new and interesting analytical techniques that enable the detection of an array of metabolites in a single assay and open new avenues for diagnostics and drug discovery. By identifying and targeting the key link in altered glucose metabolism, specific and even individualized therapeutic strategies for gastric cancer may be developed.
This article will review the recent studies on glucose metabolism in gastric cancer and particularly the key applications of glucose metabolism in gastric cancer surveillance, diagnosis and therapy.
In 1956, Otto Warburg initially observed that cancer cells generally undergo glycolysis instead of oxidative phosphorylation for energy, compared with non-neoplastic cells. The metabolic phenomenon is well known as aerobic glycolysis or the “Warburg effect”[10]. Based on the results of “Warburg effect”, increased glucose consumption, increased glycolytic activity and the accumulation of lactic acid are critical hallmarks of cancer cells[11,12]. Compared with normal cells that mainly generate energy via mitochondrial oxidative phosphorylation, cancer cells predominantly obtain energy via increased glycolysis even under aerobic conditions. Converting glucose into lactate via glycolysis is inefficient in generating ATP, but it produces a large number of intermediate products driving cell proliferation. Therefore, increasing glucose consumption, leading to anaerobic glycolysis, is believed to provide an evolutionary advantage to cancer cells[13]. The accumulation of lactic acid causes acidic microenvironment, and has a protective effect on tumor cells. Lactic acid induces the expression of glycolytic enzymes in tumor cells, such as 6-phosphofructokinase1 (PFK1) to enhance the supply of ATP, and resist cellular apoptosis and promote metastasis[14]. In addition, lactic acid promotes tumor angiogenesis, providing a suitable microenvironment for tumor development and metastasis.
A number of studies have confirmed the association between obesity and gastric cancer[15-19]. An et al[20] confirmed the relationship between glucose metabolism, diabetes and gastric cancer by observing improved glucose metabolism after treatment of gastric cancer. A higher fasting serum glucose level significantly increased the incidence of gastric cancer in Helicobacter pylori (H. pylori)-seropositive patients nearly 3.5-4.2 fold[21], suggesting that hyperglycemia may be an important cofactor in H. pylori-mediated gastric carcinogenesis[22,23]. Song et al[24] used gas chromatography/mass spectrometry (GC/MS) to analyze the tissue metabolites of gastric cancer patients and healthy controls. The GC/MS revealed that several intermediate products of aerobic glycolytic pathways, such as fumaric acid and alpha-ketoglutaric acid increase significantly in cancer tissues than in the normal mucosa, suggesting that altered glucose metabolism may be an important parameter in distinguishing gastric cancer cells from normal cells. Similarly, abnormal glucose metabolism was observed by other researchers in gastric cancer tissue[25-28].
Ikeda et al[29] demonstrated that the serum levels of 3-hydroxypropionic acid and pyruvic acid were upregulated in gastric cancer. Therefore, abnormal glucose metabolism may be related to tumor growth involving aggressive cancer cell proliferation, which requires a lot of energy, possibly causing altered serum levels of a few intermediate metabolites. Serum metabolic profiling has a great potential role in identifying gastric cancer and the underlying metabolic mechanisms[30].
Chen et al[31] used capillary electrophoresis-mass spectrometry based on moving reaction boundary (MRB-CE-MS) to investigate the metabolomics of gastric cancer patients’ urinary samples to search for possible tumor biomarkers. They found that lactic acid was remarkably increased, while citric acid, malic acid, and succinate were significantly decreased in patients with gastric cancer compared with controls, suggesting that glycolysis is upregulated while tricarboxylic acid cycle is decreased in gastric cancer[31]. The results implied that urinary metabolic profiles based on MRB-CE-MS analysis were useful in clinical diagnosis and prognosis of gastric cancer patients, consistent with findings from other studies[32-36].
About 80 years after Warburg presented his hypothesis on aberrant glucose metabolism in cancer cells, his viewpoint has been confirmed using positron emission tomography (PET) with the glucose analog tracer in clinical oncology. The potential genetic, epigenetic and proteomic mechanisms underlying the relationship between glucose metabolism and cancer have only been partially elucidated.
Carcinogenesis is due to proto-oncogene activation and tumor suppressor gene inactivation, which are closely associated with glucose metabolism. As a proto-oncogene, Myc plays an important role in glucose metabolism by enhancing the expression of glycolytic enzymes including glucose transporter 1 (GLUT1)[37], lactate dehydrogenase A (LDHA)[38,39] and pyruvate Kinase M2 (PKM2)[40]. Inactivation of p53, a well-known tumor suppressor, directly mediates the Warburg effect. In many cancers, p53 loss was observed to promote glucose flux via glycolytic pathway and reduced oxidative phosphorylation[40]. The p53 protein increases oxidative phosphorylation and decreases glycolysis via downregulation of GLUT1, GLUT3, and GLUT4 expression[41] and inactivation of glycolytic enzymes, such as phosphoglycerate mutase (PGM)[42]. Recently, we studied the role of Klotho, an anti-oncogene, in gastric cancer and found that restoration of Klotho gene expression could remarkably inhibit cell proliferation and induce apoptosis in gastric cancer cells by downregulating the phosphorylation levels of IGF-1R, IRS-1, PI3K, Akt, and mTOR proteins. In the process, it may be associated with altered glucose metabolism, which requires further research[43].
The family of glucose transporters (Gluts), which control the glucose transport across the plasma into the cytosol, play a critical role in glucose metabolism[44-46]. Increasing evidence shows that Gluts, especially the class I Gluts (1-4), play an key role in cancer glucose metabolism and cancer progression, such as in lung tumor[47], breast cancer[48], and bladder cancer[49]. Recently, Shimada et al[50] reported that Glut-3 and Glut-1 expression were positive in benign gastric schwannoma with a high FDG uptake, but Glut-2 and Glut-4 expression were negative. 18F-FDG uptake in primary gastric lymphoma is also related to GLUT1 expression[51]. Alakus et al[52] investigated GLUT-1 expression in 35 patients with gastric cancer, who underwent FDG-PET, and suggested that FDG uptake in gastric cancer is associated with GLUT-1 expression and that low FDG uptake in signet-ring cell carcinoma is due to the low expression of GLUT-1 in this histological subtype. Yamada et al[53] also observed that GLUT-1 expression occurred from an early cancer stage and was the most influential factor underlying the degree of FDG uptake in gastric carcinoma. FDG uptake correlated with GLUT-1 expression, responding to glucose metabolism, may serve as a prognostic biomarker of gastric cancer[53]. However, the study of Takebayashi et al[54] showed no connection between FDG standardized uptake value (SUV) and GLUT-1 expression in gastric cancer. Currently, evidence on the role of Gluts in glucose metabolism in gastric cancer is still limited.
Several other glycolytic enzymes, including glucose-6-phosphate dehydrogenase (G6PD)[55], hexokinase (HK)-II[56,57] and pyruvate kinase M2 (PKM2)[58,59] , have been confirmed to participate in the carcinogenesis and predict the progression of gastric cancer. G6PD is involved in the normal processing of carbohydrates by converting glucose into ribose-5-phosphate, which is the first key step in glycolysis. Overexpression of G6PD in gastric cancer tissues is significantly correlated with progression of gastric cancer. Increasing G6PD levels in gastric cancer may enhance the level of NAPDH which protects cells from DNA damage induced by reactive oxygen species (ROS)[55]. Hexokinases catalyze the first phosphorylation step of glycosis, to produce glucose-6-phosphate. HK-II is upregulated in many human cancers associated with enhanced aerobic glycolysis, the Warburg effect. HK-II was overexpressed in gastric cancer with worse prognosis[57]. Unlike HK-I that is predominant in gastric polyps and normal mucosa, a significant elevation of HK-II was found in gastric cancer, Changes in the hexokinases isoenzymes composition in gastric mucosa with intestinal metaplasia were expressed to a lesser degree but with similar likelihood of cancer[60]. HK-II, as a component of survival signaling nexus, integrates glucose metabolism and cell survival through Akt/mTOR pathways. It can positively regulate glucose starvation-induced autophagy through TORC1 inhibition[61]. Pyruvate kinase M2 (PKM2) is another glycolytic enzyme, which controls the final rate-limiting step of glycolysis by catalyzing the dephosphorylation of phosphoenolpyruvate (PEP) to pyruvate and is overexpressed in many human cancers[62]. Recent studies have indicated that PKM2 was overexpressed in gastric cancer and associated with tumor size, depth of invasion and lymph node metastasis[59,63]. The knockdown of PKM2 partially affected the stability of NF-κB subunit p65 in gastric cancer cells, suggesting that post-translational regulation of p65 by PKM2 may be a plausible mechanism correlated with cell proliferation[64]. Another study demonstrated that the PKM2 expression, E-cadherin expression, and ERK1/2 phosphorylation were correlated with each other in gastric cancer cells, which suggested an important connection between PKM2 and E-cadherin in the motility and invasion of gastric cancer cell stimulated by EGFR[65].
Hypoxia-inducible factor pathway: One of the common explanations for enhanced glycolysis in cancer cells is cancer tissue hypoxia, attributed to the rapid growth of cancer cells[66]. Hypoxia is now recognized as a key factor in carcinogenesis, and Hypoxia-inducible factor (HIF)-1 is a critical transcription factor of the HIF pathway involved in both sensing and responding to changes in cellular oxygen, which aids in the survival of cells in hypoxic microenvironment[67,68]. Increasing evidence shows that HIF-1 and HIF pathway may mediate gastric carcinogenesis. Generally HIF-1 is not or minimally expressed in the normal gastric mucosa from patients with gastric cancer, peptic ulcer or dyspepsia[69,70]. Recently, Lin et al[71] conducted a systematic review of the literature and meta-analysis to investigate the role of HIF-1α in gastric cancer. Of the nine studies including 1103 subjects, HIF-1α positive expression was observed in half the patients and always indicated poor prognosis for patients with gastric cancer. Activation of the Ras-MAPK signal transduction pathway and PI3K-AKT-mTOR signaling, and loss of tumor suppressor proteins, such as PTEN and p53, elevated HIF-1α expression. HIF-1α directly stimulates glycolysis by activating the expression of glucose transporters and several key glycolytic enzymes, such as HK, PKM2 and LDH-A. The HIF-1α-dependent pathway increases glycolysis and inhibits mitochondrial O2 consumption, then promoting tumor cell survival[72,73].
Insulin signaling pathway: Another important signaling pathway involved in glucose metabolism is insulin signaling pathway. Suppressing glucose production in the liver and enhancing glucose uptake in the insulin-sensitive tissues of the human body is well known as the classic action of insulin mediating glucose homeostasis. Insulin is also implicated in cellular activation and angiogenesis mediated by the activation of signaling of the insulin receptor (IR), insulin growth factor (IGF)-1, IGF2 and the IGF-1R[74,75]. Increased IGF signaling is associated with many cancers[74-76]. In addition, downregulation of the IGF-1 receptor expression and reduced signaling have been found to inhibit tumor growth[77]. Increasing IGF-I expression was observed in gastric tumors progressing from benign proliferative lesions to malignant lesions[78]. IGF-I can induce epithelial-to-mesenchymal transition (EMT) which is involved in the metastasis of numerous cancers, by activating a PI3K/Akt-GSK-3β-ZEB2 signaling pathway in gastric cancer BGC-823 cells[79]. Li et al[76] and Min et al[80] found that IGF-IR signaling promoted tumor growth in gastric cancer. IGF-1R blockade reduced gastric tumor growth in vivo and in vitro by inhibiting both angiogenesis and lymphangiogenesis, attributed to the decreasing activity of both protein kinase B (Akt) and mitogen-activated protein kinase (MAPK). IGF-1R expression in gastric cancer was correlated with lymph node metastasis, poor prognosis and high histological malignancy grade, and may play an important role in tumor growth and metastasis via the lymphatic pathway[81].
PI3K-Akt-mTOR pathway: The PI3K-Akt-mTOR pathway is currently a widely studied intracellular signaling pathway. It is directly associated with cellular quiescence, proliferation, cancer, and longevity. Recent studies showed that the PI3K-Akt-mTOR pathway was activated in gastric cancer and activation of the pathway was correlated with metastasis, poor prognosis and lower survival in gastric cancer[82,83]. In addition, activation of this pathway promoted glycolysis and inhibited autophagy[84]. Akt expression directly increases the surface translocation of glucose transporters and enhances aerobic glycolysis by prompting HK-II binding to voltage-dependent anion channel. (VDAC) at the outer mitochondrial membrane[85]. PI3K/Akt also increased fatty acid synthesis in cancer cells by suppressing mitochondrial acid fatty oxidation and promoting a metabolic phenotype supporting cancer cell growth and proliferation in the absence of glucose by oxidizing fatty acid[86]. Upregulation of the PI3K-Akt-mTOR pathway and increased glucose consumption via glycolysis offer evolutionary advantages to cancer cells in normoxia as well as hypoxia.
In general, the regulation of glucose metabolism in carcinogenesis is a multi-factor, multi-step process. In their review, Smolková et al[87] presented the wave hypothesis of metabolic regulation during carcinogenesis, which consisted of four waves. First, the fundamental reprogramming of gene expression or initiation by stem cells establishes the conditions conducive to cancer cell proliferation. Second, subsequent responses to microenvironmental conditions cause a typical Warburg effect. Third, aglycemia and nutrient shortage due to rapid cell growth during malignancy, stimulate glutaminolysis, which may influence restoration of suppressed mitochondrial biogenesis, leading to oxidative phosphorylation (OXPHOS)-dependent cancer cells. The fourth wave of gene reprogramming entails retrograde signaling from revitalized mitochondria.
Based on the increased glucose uptake in cancer cells, PET/CT scan can reflect cancer cell glucose metabolism using 18F-2-fluoro-2-deoxy-D-glucose (18F-FDG) as a tracer and has been widely used in the diagnosis and monitoring of human cancers. 18F-FDG is the most commonly used radiolabeled glucose analog in clinical practice. Currently, gastroscopic biopsy and histopathological examination are the gold standard of diagnosis of gastric cancer. In recent years, PET/CT, integrating images from FDG-PET with CT, have been used to detect gastric cancer. Compared with contrast-enhanced CT (CECT) and endoscopic ultrasonography, PET/CT does not offer the advantages of sensitivity and accuracy, and therefore, FDG-PET/CT scans are not indicated in routine staging of gastric cancer[88]. Nevertheless, due to its high specificity, PET/CT is useful when CECT findings were equivocal and in the detection of distant lymph node metastasis[89].
Several factors influence the visibility of PET/CT in gastric carcinoma. PET/CT imaging is based on increased glucose metabolism in gastric cancer. FDG avidity depends on tumor histologic subtype. Low FDG uptake is more often seen in diffuse type histology (mucinous, signet ring and poorly differentiated) compared with the intestinal subtype, which depends on GLUT-1 expression. The low GLUT-1 expression may be lead to low FDG uptake in signet-ring cell carcinoma of gastric cancer[52,53]. The maximum standardized uptake value [SUV(max)] of PET/CT is significantly correlated with tumor size, and lower FDG uptake is often found in early gastric carcinoma which is likely due to less total cancer cells in the primary lesions[53,54]. The motility and physiological uptake of 18F-FDG in the stomach also influence the accuracy of PET/CT in diagnosis of gastric cancer. Stomach distension to increase gastric volume with water or milk can reduce physiological gastric FDG uptake, display the lesions more clearly and significantly improve the diagnostic accuracy[90,91].
Although PET/CT is not recommended in the primary detection of gastric cancer due to its poor sensitivity, FDG-PET shows better results in the evaluation of biological aggressiveness and/ or patient prognosis in gastric cancer[92,93]. 18F-FDG uptake is an independent and significant prognostic indicator of tumor recurrence in gastric cancer. Lee et al[92] also investigated the role of 18F-FDG PET in gastric cancer prognosis based on histopathological subtypes and found that patients with negative 18F-FDG tumor uptake showed better recurrence-free survival than those with positive 18F-FDG tumor uptake in the subgroup of patients with gastric adenocarcinoma, while the opposite findings were obtained in the subgroup patients with signet-ring cell carcinoma and mucinous adenocarcinoma.
A higher SUVmax of 18F-FDG PET/CT was linked to the presence of microsatellite instability (MSI) in gastric cancer[94]. Gastric cancers with MSI tend to show less lymph node metastasis and manifest favorable prognosis[95]. The high SUVmax of 18F-FDG PET/CT showed poor prognosis in gastric cancer. The SUVmax more than 3.8 indicated increasingly aggressive behavior, elevated postoperative recurrence and shorter relapse-free survival in gastric signet-ring cell carcinoma[96]. The degree of 18F-FDG uptake in gastric cancer predicts histologically positive lymph nodes and non-curative surgery. The sensitivity, specificity and accuracy of the diagnosis of metastatic lymph node were 73.5%, 74.5% and 74.1%, respectively, using the SUVmax cutoff of 3.75 or greater. When the SUVmax was defined as 4.35 or more for metastatic lymph nodes to predict non-curative surgery, the sensitivity and specificity were 58.8% and 91.6%, respectively, which were higher than those obtained with CT scan. Therefore, pretreatment PET/CT may be helpful in optimizing surgical strategy[96].
Postoperative routine follow-up of gastric cancer is important to the surveillance of recurrence. The conventional follow-up using computed tomography (CT) and endoscopy, cannot frequently detect recurrence before symptom development. PET/CT shows good specificity for asymptomatic advanced gastric cancer and provides useful information for the clinical management of patients with suspected gastric cancer recurrence[97]. However, prudence should be exercised with the incidental findings of PET/CT, most of which were benign with additional investigations associated with high cost[98].
Despite adequate surgery with radical lymphadenectomy, the prognosis of advanced gastric cancer is still poor. Since the early 1990s, neoadjuvant therapy gained importance for the treatment of locally advanced or initially unresectable GC[99]. Currently, measurement of changes in morphology using different imaging modalities is still the main approach to evaluate the response to neoadjuvant therapy. However, alterations in glucose metabolism always precede changes in morphological changes. The response to neoadjuvant therapy initially manifests in altered glucose uptake, demonstrated with 18F-FDG PET. In fact, a dual modality PET-CT has been recommended for early assessment of therapeutic response in GIST patients treated with imatinib[100]. In gastric cancer, changes in FDP uptake occur early during the course of neoadjuvant therapy, which is significantly related with histopathological responses, and a complete metabolic response in FDP-PET always suggest favorable prognosis[101]. It is still a challenge to distinguish the complete histopathological remission after neoadjuvant therapy by PET/CT. Different histologic subtypes may interfere with the evaluation of PET/CT and the best time to undergo post-treatment PET/CT is still unclear.
PET/CT is a powerful, noninvasive metabolic imaging modality for detecting many human tumors. However, detection of gastric cancer by PET/CT may seem less than ideal, because FDP uptake is strongly related to tumor size and histopathological subtype[102]. In order to improve the sensitivity and specificity of PET/CT in evaluating gastric cancer, other tracers target more specific biological processes, such as proliferation (18F-3′-fluoro-3′-deoxy-L-thymidine; 18F-FLT), tumor hypoxia (18F-fluoromisonidazol; 18F-FMISO) and phospholipid metabolism (radioactively labeled choline derivates)[103-105]. 18F-FLT, as a substrate for thymidine kinase 1, is a new PET tracer with the potential ability to accumulate in proliferating tissues and malignant tumors[106]. A higher accumulation of 18F-FLT was reported in gastric cancer than in normal gastric mucosa. 18F-FLT uptake is significantly correlated with gastric cancer differentiation and cellular density[107]. 18F-FLT PET was more sensitive than 18F-FDG PET in imaging gastric cancer, especially in tumors frequently presenting with or without low 18F-FDG uptake, and may improve early evaluation of response to neoadjuvant treatment[103]. However, F-FLT PET/CT imaging is not recommended for pre-treatment assessment of metastatic gastric cancer as it does not show significant advantages in evaluating liver and bone metastases, compared with 18F-FDG PET/CT imaging[108].
Currently, gastroscopy still represents the gold standard for diagnosis of gastric cancer. Despite its uncomfortable and invasive features, gastroscopy is widely used in the surveillance, early screening and follow-up of gastric cancer. Over the past few years, several tumor serum biomarkers have been used as novel non-invasive tools for early diagnosis of gastric cancer. However, due to low specificity and sensitivity, current serum biomarkers such as CEA and carbohydrate antigens are not as effective as other screening methods.
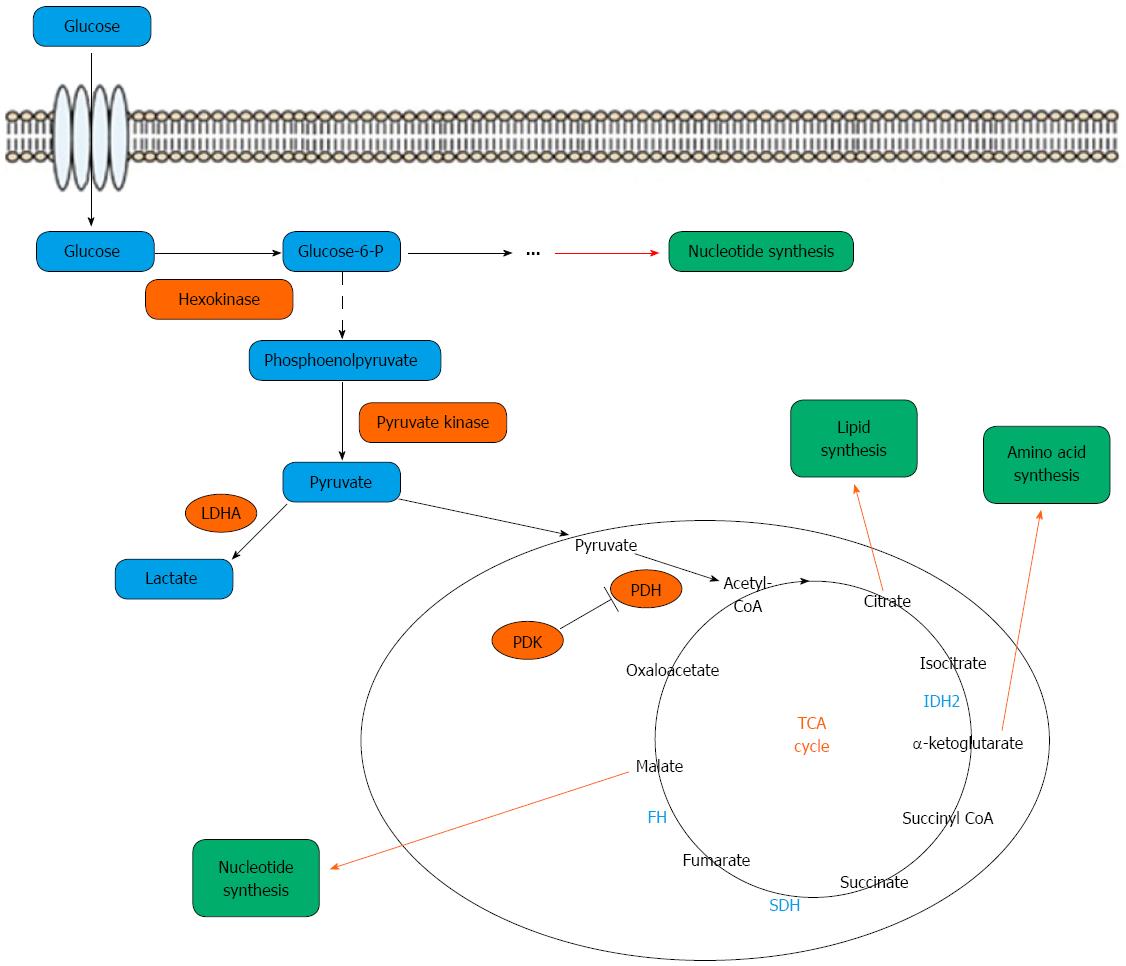
Recently, serum and urine metabolomic studies of gastric cancer based on the use of highly sensitive detection techniques may present a novel opportunity to seek potential new biomarkers for early screening of asymptomatic gastric cancer and its follow-up. Metabolomics is defined as a quantitative description of all endogenous low-molecular-weight components (< 1 kDa) in a biological sample, such as tissue, urine or plasma, and aims to diagnose various diseases by analyzing the data[109]. The small molecule endogenous metabolites are mainly composed of the intermediate products produced by the four metabolic cycles, which include the glucose metabolism, lipid metabolism, amino acid metabolism and nucleic acid metabolism, in which glucose metabolism is the core (Figure 1)[110]. The intermediate metabolites are important in biological systems and are promising candidates for understand disease phenotypes[111]. Metabolomic studies, comparing the metabolite profiles of cancer cells vs normal cells, offer an opportunity to identify the changes in metabolic pathways, which prompt carcinogenesis. Compared with genomics, transcriptomics and proteomics, metabolomics provides terminal molecular data of the biological system which may be an effective way to elucidate the phenotypic changes associated with cancer. Recently, metabolomic studies have been successfully conducted in gastric cancer[24-36] and other human cancers[112-115].
Metabolomics approaches in gastric cancer may be applied largely in three ways. First, specific metabolites responsible for phenotypes associated with cancer-related mutations should be identified. Second, the common metabolites with altered levels in gastric cancer cells compared with normal cells, need to be found. Third, the response of cancer metabolism to environmental changes needs investigation[28,29]. Advances in the highly sensitive metabolomic methods and data analysis techniques may facilitate such applications in a single study[116]. Metabolomic profiles may offer a chance to identify the potential biomarkers for early diagnosis, prognosis, drug target identification and treatment response. Recently, several metabolites were suggested as diagnostic and prognostic biomarkers for gastric cancer[117,118]. Gastric carcinogenesis is a complex phenomenon involving multiple epigenetic and genetic factors including several genetic, environmental and infectious agents causing a cumulative effect in the early stages. The model of gastric carcinogenesis is well known and includes the following sequential stages: chronic atrophic gastritis (CAG), intestinal metaplasia (IM), gastric dysplasia (DYS) and finally gastric cancer (GC). Plasma metabolomic studies, in which fifteen identified metabolites were quantitatively detected, showed unique metabolic profiles in the different stages of GC. The metabolic phenotype of chronic superficial gastritis (CSG) is significantly different from CAG, IM, DYS and GC, whose plots clustered closely. A similar metabolic pattern was shown in IM and GC[119]. The discriminative metabolites characterizing the different stages of GC may be widely used in gastric cancer screening and early diagnosis combined with endoscopy. As previously mentioned, several serum metabolomic studies in GC suggested significant metabolic differences between cancer and control groups, suggesting potential biomarkers for the early diagnosis of GC. Kim et al[33] studied urinary metabolomic biomarkers of gastric cancer in mouse models and found the presence of significant endogenous metabolic differences between tumor-bearing mice and controls. The study indicated that trimethylamine oxide (TMAO), 3-indoxylsulfate, hippurate, and citrate might serve as useful urinary biomarkers for the detection of gastric cancer in a mouse model. Jung et al[35] demonstrated that metabolomic changes in amino acid and lipid metabolism of urine samples resembled those in gastric cancer tissue, and were highly accurate in predicting gastric cancer with a much higher sensitivity than carbohydrate antigen 19-9 and CEA. Further, 4-hydroxyphenylacetate, alanine, phenylacetylglycine, mannitol, glycolate, and arginine levels were significantly related to T stage of gastric cancer. Compared with serum and urine samples, tissue samples require invasive approaches such as endoscopy and surgery, which limits their application in the screening and early diagnosis of gastric cancer. However, identification of new biomarkers simultaneously in tissue and serum or urine samples is helpful. Tissue metabolic markers in gastric cancer were identified by some studies[23,24]. Integrated the recent studies on the profiling of glucose metabolites in gastric cancer, lactate and fumarate were recognized as the most commonly biomarkers for gastric cancer screening and diagnosis[120].
Recent advances in metabolomics have offered new avenues to predict gastric cancer metastasis. Hur et al[28] analyzed the levels of Krebs cycle components in gastric cancer tissues and found that the levels of pyruvic acid, lactic acid and ketone bodies were associated with histopathology. In particular, the levels of ketone bodies were significantly higher in cancer tissues with differentiated tumors than in undifferentiated tumors. Wu et al[25] found that the levels of phenanthrenol and butanoic acid were significantly decreased in invasive cancers (T3/T4) compared with non-invasive cancers (T1/T2). Compared with glucose metabolites, amino acid and lipid metabolites seem to be more potential in predicting gastric cancer prognosis. Using animal models of human gastric cancer, Chen et al[121] demonstrated that proline and serine metabolism play an important role in metastasis, and may be used in predicting gastric cancer metastasis and progression. Further, Hu et al[34] investigated urinary metabolite profiling to identify possible biomarkers in gastric cancer metastasis. They found that the levels of alanine, glycerol and L-proline were lower and the level of myo-inositol was higher in the metastasis group. These urinary metabolites may be a potential prognostic biomarker for gastric cancer metastasis.
Predicting drug response: Currently, chemotherapy has become a first-line therapy for advanced gastric cancer. However, chemotherapy is not the gold standard due to its lower effectiveness compared with its role in colorectal and breast cancers. Chemoresistance is a major challenge. Recent studies suggested that metabolomics may play an important role in investigating cellular responses to chemotherapy of cancer[122-126]. Morvan et al[124] investigated metabolic changes associated with tumor response to chloroethyl nitrosourea (CENU), an anticancer agent, in two tumor models in vivo and observed the activation of metabolic pathways of DNA repair and adaptation to treatment. Metabolomics of tumor response to anticancer agents may enable identification of metabolic pathways of drug efficacy and evaluate the effectiveness of treatment. Wang et al[125] assessed metabolomic prediction of chemosensitivity in a human xenograft model of gastric cancer and determined that a series of endogenous metabolites, including 1-acyl-lysophosphatidycholines, polyunsaturated fatty acids and their derivatives, were predictive of chemosensitivity in gastric cancer. Sasada et al[126] conducted a similar metabolomic analysis to investigate the intracellular response of human gastric cancer cells to 5-fluorouracil (5-FU), and showed a dramatic alteration in the number of metabolites, especially proline, glutamate and proline dehydrogenase (PRODH), in non-5-FU-resistant cancer cells during short-term treatment with 5-FU. However, the proline and glutamate levels and PRODH mRNA expression were less affected in 5-FU-resistant cancer cells. In the future, metabolic biomarkers may play an important role in evaluating treatment response to anticancer drugs.
Enhanced glucose metabolism via aerobic glycolysis followed by lactic acid fermentation plays a predominant role in rapid energy (ATP) synthesis. However, generation of large number of metabolites contributes to an acidic micro-environment conducive to cancer proliferation. Therapeutically targeting cancer cell metabolism such as glucose metabolism is more convenient and associated with fewer side effects compared with the other biologic systems since cellular metabolic pathways represent the terminus of systems biology and control the other systems genetically.
Ketogenic diets consist of high fat, with moderate-to-low protein content, and very low carbohydrates. It reduced tumor growth and improved survival in a mouse model of malignant gastric cancer[127]. Furthermore, ketogenic diets have been suggested to increase the effects of radiochemotherapy in non-small cell lung cancer xenograft models[128]. Recent studies revealed that ketogenic diets act as adjuvant cancer therapy via mechanisms that increased oxidative stress and inhibited glucose metabolism via lipid metabolism.
Metformin, a first-line anti-diabetic drug, inhibited proliferation and induced apoptosis in cancer cells. Metformin decreased mitochondrial respiration chain activity and ATP production and induced the activation of LKB1-AMPK, causing the inhibition of Raptor-mTOR complex[129,130]. High glucose concentrations reduced the effectiveness of metformin on cancer cell proliferation and failure to maintain glucose homeostasis may promote aggressive breast cancer phenotype [129].
Tanshinone IIA, a diterpene quinone extracted from the plant Danshen, has been recently reported as an effective adjunctive reagent in the treatment of gastric cancer[131]. Lin et al[132] confirmed that TIIA treatment inhibited cell growth and the proliferation of gastric cancer by suppressing glucose metabolism in cancer cells. Another study conducted by Bhattacharya et al[133] revealed that hypoglycemia and enhanced glycolysis increased resistance to chemotherapy in gastric cancer.
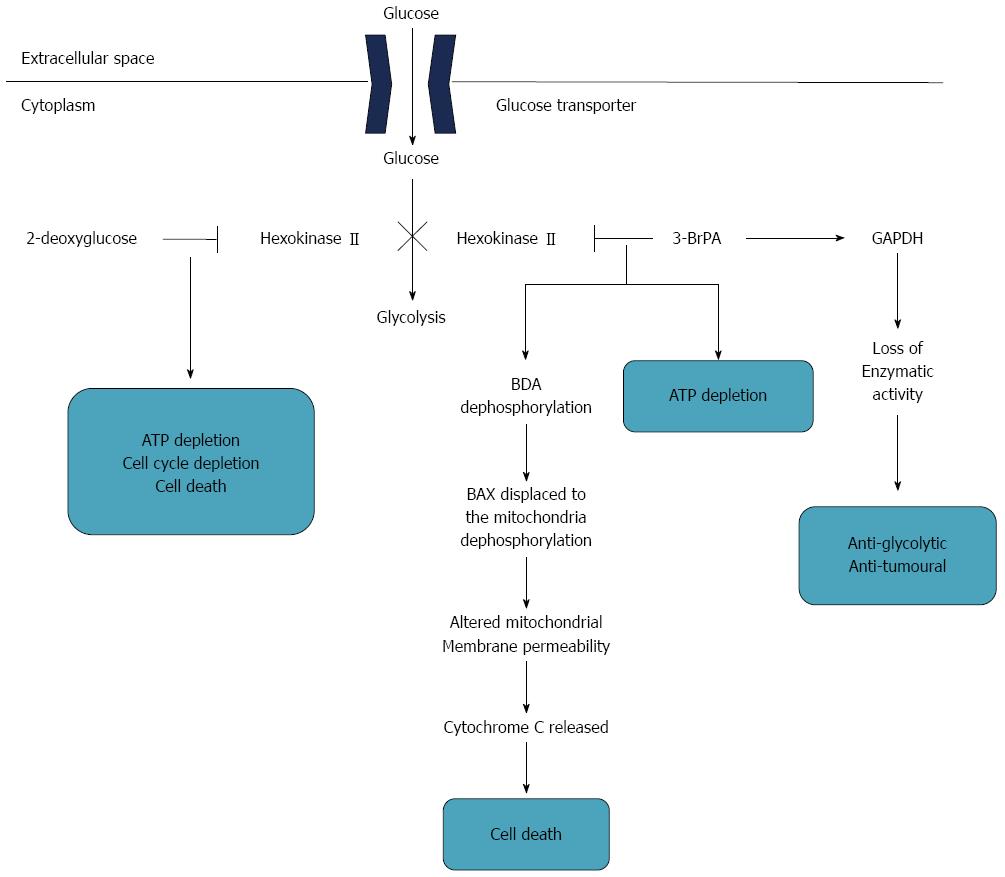
Another very promising strategy is to design gastric cancer-specific and even individualized inhibitors of all the steps of the glycolytic pathway via metabolomics studies. Granchi et al[134] reviewed recent advances of new bioactive molecules which disturb cancer glycolysis. Different kinds of small molecules that inhibit all the steps of the glycolytic pathway have been identified, such as hexokinase II (HKII), 2-deoxy-D-glucose and 3-bromopyruvate (3-BrPA), which were accepted as cancer therapeutic targets in several studies. Similar potential and promising therapy for gastric cancer was described by Ngo et al[135] (Figure 2). Hexokinase II is a key factor catalyzing the first step of glycolysis, which consists of the transfer of glucose to glucose-6-phosphate[136]. HKII is accepted as a very potential and attractive anticancer target. 2-deoxy-D-glucose, a glucose analogue, binds and inhibits HKII, resulting in cellular ATP depletion, cell cycle suppression and cell death[137]. 3-BrPA, an alkylating agent and glycolysis inhibitor and designated as an orphan drug by FDA for liver cancer, has been identified to hinder glucose metabolism by inhibiting HKII[138]. In addition, 3-BrPA inactivated the glyceraldehydes-3-phosphate dehydrogenase (GAPDH) by GAPDH pyruvylation, leading to anti-glycolytic and antitumoral effects and mediating cancer cell death[139].
Generally, most of the current glycolytic inhibitors showed only moderate efficacy when used as single agents, but in some cases demonstrated high potential when combined with other therapies. Currently, few therapeutic studies target gastric cancer metabolism. It is anticipated that further research will investigate the role of cancer-specific glycolytic inhibitors to develop effective therapeutic regimens for gastric cancer.
Altered glucose metabolism is a hallmark of gastric cancer, which provides new insights into gastric carcinogenesis and identification of biomarkers targeting specific metabolic aspects of gastric cancer. However, several hurdles remain before altered glucose metabolism is clinically used in the diagnosis and treatment of gastric cancer. The use of 18F-FDG PET is an exception. However, a major clinical application of metabolomics requires creation of spectral databases of metabolites of the normal population, similar to the cancer genomic and proteomic databases. Further, the metabolites consist of a large group of small-molecule intermediates, which are too large for analysis using currently available technology. Another challenge is related to identification of cancer-specific biomarkers for gastric cancer, since metabolites may be considered as potential biomarkers for a range of cancers. It is anticipated that further research will focus on these aspects and promote the clinical application of glucose metabolism in gastric cancer in the not-too-distant future.
P- Reviewer: Cho JY, Lee SM S- Editor: Yu J L- Editor: Filipodia E- Editor: Ma S
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25175] [Article Influence: 1936.5] [Reference Citation Analysis (3)] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11614] [Article Influence: 893.4] [Reference Citation Analysis (4)] |
| 3. | An JY, Cheong JH, Hyung WJ, Noh SH. Recent evolution of surgical treatment for gastric cancer in Korea. J Gastric Cancer. 2011;11:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. [PubMed] [Cited in This Article: ] |
| 5. | Lin LL, Huang HC, Juan HF. Discovery of biomarkers for gastric cancer: a proteomics approach. J Proteomics. 2012;75:3081-3097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Yamashita K, Sakuramoto S, Watanabe M. Genomic and epigenetic profiles of gastric cancer: potential diagnostic and therapeutic applications. Surg Today. 2011;41:24-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1055] [Cited by in F6Publishing: 1176] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 8. | Di Chiro G, DeLaPaz RL, Brooks RA, Sokoloff L, Kornblith PL, Smith BH, Patronas NJ, Kufta CV, Kessler RM, Johnston GS. Glucose utilization of cerebral gliomas measured by [18F] fluorodeoxyglucose and positron emission tomography. Neurology. 1982;32:1323-1329. [PubMed] [Cited in This Article: ] |
| 9. | Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 578] [Cited by in F6Publishing: 642] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 10. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [PubMed] [Cited in This Article: ] |
| 11. | Ngo DC, Ververis K, Tortorella SM, Karagiannis TC. Introduction to the molecular basis of cancer metabolism and the Warburg effect. Mol Biol Rep. 2015;42:819-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10702] [Cited by in F6Publishing: 10514] [Article Influence: 700.9] [Reference Citation Analysis (0)] |
| 13. | Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3445] [Cited by in F6Publishing: 3493] [Article Influence: 268.7] [Reference Citation Analysis (0)] |
| 14. | Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685-3692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 640] [Cited by in F6Publishing: 777] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 15. | Chow WH, Blot WJ, Vaughan TL, Risch HA, Gammon MD, Stanford JL, Dubrow R, Schoenberg JB, Mayne ST, Farrow DC. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150-155. [PubMed] [Cited in This Article: ] |
| 16. | Ji BT, Chow WH, Yang G, McLaughlin JK, Gao RN, Zheng W, Shu XO, Jin F, Fraumeni JF, Gao YT. Body mass index and the risk of cancers of the gastric cardia and distal stomach in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 1997;6:481-485. [PubMed] [Cited in This Article: ] |
| 17. | Kubo M, Sano T, Fukagawa T, Katai H, Sasako M. Increasing body mass index in Japanese patients with gastric cancer. Gastric Cancer. 2005;8:39-41. [PubMed] [Cited in This Article: ] |
| 18. | Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, Wu XT. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45:2867-2873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 19. | Abnet CC, Freedman ND, Hollenbeck AR, Fraumeni JF, Leitzmann M, Schatzkin A. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer. 2008;44:465-471. [PubMed] [Cited in This Article: ] |
| 20. | An JY, Kim YM, Yun MA, Jeon BH, Noh SH. Improvement of type 2 diabetes mellitus after gastric cancer surgery: short-term outcome analysis after gastrectomy. World J Gastroenterol. 2013;19:9410-9417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Yamagata H, Kiyohara Y, Nakamura S, Kubo M, Tanizaki Y, Matsumoto T, Tanaka K, Kato I, Shirota T, Iida M. Impact of fasting plasma glucose levels on gastric cancer incidence in a general Japanese population: the Hisayama study. Diabetes Care. 2005;28:789-794. [PubMed] [Cited in This Article: ] |
| 22. | Marimuthu SP, Vijayaragavan P, Moysich KB, Jayaprakash V. Diabetes mellitus and gastric carcinoma: Is there an association? J Carcinog. 2011;10:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Sheu SM, Cheng H, Kao CY, Yang YJ, Wu JJ, Sheu BS. Higher glucose level can enhance the H. pylori adhesion and virulence related with type IV secretion system in AGS cells. J Biomed Sci. 2014;21:96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Song H, Wang L, Liu HL, Wu XB, Wang HS, Liu ZH, Li Y, Diao DC, Chen HL, Peng JS. Tissue metabolomic fingerprinting reveals metabolic disorders associated with human gastric cancer morbidity. Oncol Rep. 2011;26:431-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Wu H, Xue R, Tang Z, Deng C, Liu T, Zeng H, Sun Y, Shen X. Metabolomic investigation of gastric cancer tissue using gas chromatography/mass spectrometry. Anal Bioanal Chem. 2010;396:1385-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Cai Z, Zhao JS, Li JJ, Peng DN, Wang XY, Chen TL, Qiu YP, Chen PP, Li WJ, Xu LY. A combined proteomics and metabolomics profiling of gastric cardia cancer reveals characteristic dysregulations in glucose metabolism. Mol Cell Proteomics. 2010;9:2617-2628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 27. | Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009;69:4918-4925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 687] [Cited by in F6Publishing: 717] [Article Influence: 47.8] [Reference Citation Analysis (1)] |
| 28. | Hur H, Paik MJ, Xuan Y, Nguyen DT, Ham IH, Yun J, Cho YK, Lee G, Han SU. Quantitative measurement of organic acids in tissues from gastric cancer patients indicates increased glucose metabolism in gastric cancer. PLoS One. 2014;9:e98581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Ikeda A, Nishiumi S, Shinohara M, Yoshie T, Hatano N, Okuno T, Bamba T, Fukusaki E, Takenawa T, Azuma T. Serum metabolomics as a novel diagnostic approach for gastrointestinal cancer. Biomed Chromatogr. 2012;26:548-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Song H, Peng JS, Dong-Sheng Y, Yang ZL, Liu HL, Zeng YK, Shi XP, Lu BY. Serum metabolic profiling of human gastric cancer based on gas chromatography/mass spectrometry. Braz J Med Biol Res. 2012;45:78-85. [PubMed] [Cited in This Article: ] |
| 31. | Chen JL, Fan J, Lu XJ. CE-MS based on moving reaction boundary method for urinary metabolomic analysis of gastric cancer patients. Electrophoresis. 2014;35:1032-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Ozcan S, Barkauskas DA, Renee Ruhaak L, Torres J, Cooke CL, An HJ, Hua S, Williams CC, Dimapasoc LM, Han Kim J. Serum glycan signatures of gastric cancer. Cancer Prev Res (Phila). 2014;7:226-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Kim KB, Yang JY, Kwack SJ, Park KL, Kim HS, Ryu do H, Kim YJ, Hwang GS, Lee BM. Toxicometabolomics of urinary biomarkers for human gastric cancer in a mouse model. J Toxicol Environ Health A. 2010;73:1420-1430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Hu JD, Tang HQ, Zhang Q, Fan J, Hong J, Gu JZ, Chen JL. Prediction of gastric cancer metastasis through urinary metabolomic investigation using GC/MS. World J Gastroenterol. 2011;17:727-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 44] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Jung J, Jung Y, Bang EJ, Cho SI, Jang YJ, Kwak JM, Ryu do H, Park S, Hwang GS. Noninvasive diagnosis and evaluation of curative surgery for gastric cancer by using NMR-based metabolomic profiling. Ann Surg Oncol. 2014;21 Suppl 4:S736-S742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Zhang Y, Ren H, Jiang Y, Gao YF, Liu SY. Urinary metabonomics of stomach cancer assessed by rapid resolution liquid chromatography/time-of-fight mass spectrometry. Chin Med J (Engl). 2013;126:1930-1933. [PubMed] [Cited in This Article: ] |
| 37. | Buller CL, Loberg RD, Fan MH, Zhu Q, Park JL, Vesely E, Inoki K, Guan KL, Brosius FC. A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am J Physiol Cell Physiol. 2008;295:C836-C843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 38. | Ma J, Liu W, Guo H, Li S, Cao W, Du X, Lei S, Hou W, Xiong L, Yao L. N-myc downstream-regulated gene 2 expression is associated with glucose transport and correlated with prognosis in breast carcinoma. Breast Cancer Res. 2014;16:R27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Qiu H, Jackson AL, Kilgore JE, Zhong Y, Chan LL, Gehrig PA, Zhou C, Bae-Jump VL. JQ1 suppresses tumor growth through downregulating LDHA in ovarian cancer. Oncotarget. 2015;6:6915-6930. [PubMed] [Cited in This Article: ] |
| 40. | Wu H, Li Z, Yang P, Zhang L, Fan Y, Li Z. PKM2 depletion induces the compensation of glutaminolysis through β-catenin/c-Myc pathway in tumor cells. Cell Signal. 2014;26:2397-2405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Watanabe M, Naraba H, Sakyo T, Kitagawa T. DNA damage-induced modulation of GLUT3 expression is mediated through p53-independent extracellular signal-regulated kinase signaling in HeLa cells. Mol Cancer Res. 2010;8:1547-1557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 42. | Ruiz-Lozano P, Hixon ML, Wagner MW, Flores AI, Ikawa S, Baldwin AS, Chien KR, Gualberto A. p53 is a transcriptional activator of the muscle-specific phosphoglycerate mutase gene and contributes in vivo to the control of its cardiac expression. Cell Growth Differ. 1999;10:295-306. [PubMed] [Cited in This Article: ] |
| 43. | Xie B, Zhou J, Shu G, Liu DC, Zhou J, Chen J, Yuan L. Restoration of klotho gene expression induces apoptosis and autophagy in gastric cancer cells: tumor suppressive role of klotho in gastric cancer. Cancer Cell Int. 2013;13:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Krzeslak A, Wojcik-Krowiranda K, Forma E, Jozwiak P, Romanowicz H, Bienkiewicz A, Brys M. Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers. Pathol Oncol Res. 2012;18:721-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 45. | Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654-662. [PubMed] [Cited in This Article: ] |
| 46. | Augustin R. The protein family of glucose transport facilitators: It’s not only about glucose after all. IUBMB Life. 2010;62:315-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 47. | Younes M, Brown RW, Stephenson M, Gondo M, Cagle PT. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer. 1997;80:1046-1051. [PubMed] [Cited in This Article: ] |
| 48. | Younes M, Brown RW, Mody DR, Fernandez L, Laucirica R. GLUT1 expression in human breast carcinoma: correlation with known prognostic markers. Anticancer Res. 1995;15:2895-2898. [PubMed] [Cited in This Article: ] |
| 49. | Younes M, Juarez D, Lechago LV, Lerner SP. Glut 1 expression in transitional cell carcinoma of the urinary bladder is associated with poor patient survival. Anticancer Res. 2001;21:575-578. [PubMed] [Cited in This Article: ] |
| 50. | Shimada Y, Sawada S, Hojo S, Okumura T, Nagata T, Nomoto K, Tsukada K. Glucose transporter 3 and 1 may facilitate high uptake of 18F-FDG in gastric schwannoma. Clin Nucl Med. 2013;38:e417-e420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Watanabe Y, Suefuji H, Hirose Y, Kaida H, Suzuki G, Uozumi J, Ogo E, Miura M, Takasu K, Miyazaki K. 18F-FDG uptake in primary gastric malignant lymphoma correlates with glucose transporter 1 expression and histologic malignant potential. Int J Hematol. 2013;97:43-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Alakus H, Batur M, Schmidt M, Drebber U, Baldus SE, Vallböhmer D, Prenzel KL, Metzger R, Bollschweiler E, Hölscher AH. Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl Med Commun. 2010;31:532-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 53. | Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med. 2006;20:597-604. [PubMed] [Cited in This Article: ] |
| 54. | Takebayashi R, Izuishi K, Yamamoto Y, Kameyama R, Mori H, Masaki T, Suzuki Y. [18F]Fluorodeoxyglucose accumulation as a biological marker of hypoxic status but not glucose transport ability in gastric cancer. J Exp Clin Cancer Res. 2013;32:34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Wang J, Yuan W, Chen Z, Wu S, Chen J, Ge J, Hou F, Chen Z. Overexpression of G6PD is associated with poor clinical outcome in gastric cancer. Tumour Biol. 2012;33:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Qiu MZ, Han B, Luo HY, Zhou ZW, Wang ZQ, Wang FH, Li YH, Xu RH. Expressions of hypoxia-inducible factor-1α and hexokinase-II in gastric adenocarcinoma: the impact on prognosis and correlation to clinicopathologic features. Tumour Biol. 2011;32:159-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Rho M, Kim J, Jee CD, Lee YM, Lee HE, Kim MA, Lee HS, Kim WH. Expression of type 2 hexokinase and mitochondria-related genes in gastric carcinoma tissues and cell lines. Anticancer Res. 2007;27:251-258. [PubMed] [Cited in This Article: ] |
| 58. | Hur H, Xuan Y, Kim YB, Lee G, Shim W, Yun J, Ham IH, Han SU. Expression of pyruvate dehydrogenase kinase-1 in gastric cancer as a potential therapeutic target. Int J Oncol. 2013;42:44-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 59. | Yin L, Wang X, Luo C, Liu H, Zhang L, Zhang H, Zhang Y. The value of expression of M2-PK and VEGF in patients with advanced gastric cancer. Cell Biochem Biophys. 2013;67:1033-1039. [PubMed] [Cited in This Article: ] |
| 60. | Bassalyk LS, Ljubimova NV. Hexokinase isoenzymes in the diagnosis of gastric and esophageal neoplasms. Neoplasma. 1987;34:319-324. [PubMed] [Cited in This Article: ] |
| 61. | Roberts DJ, Tan-Sah VP, Ding EY, Smith JM, Miyamoto S. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol Cell. 2014;53:521-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 62. | Eigenbrodt E, Basenau D, Holthusen S, Mazurek S, Fischer G. Quantification of tumor type M2 pyruvate kinase (Tu M2-PK) in human carcinomas. Anticancer Res. 1997;17:3153-3156. [PubMed] [Cited in This Article: ] |
| 63. | Lim JY, Yoon SO, Seol SY, Hong SW, Kim JW, Choi SH, Cho JY. Overexpression of the M2 isoform of pyruvate kinase is an adverse prognostic factor for signet ring cell gastric cancer. World J Gastroenterol. 2012;18:4037-4043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 61] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 64. | Kwon OH, Kang TW, Kim JH, Kim M, Noh SM, Song KS, Yoo HS, Kim WH, Xie Z, Pocalyko D. Pyruvate kinase M2 promotes the growth of gastric cancer cells via regulation of Bcl-xL expression at transcriptional level. Biochem Biophys Res Commun. 2012;423:38-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Wang LY, Liu YP, Chen LG, Chen YL, Tan L, Liu JJ, Jazag A, Ren JL, Guleng B. Pyruvate kinase M2 plays a dual role on regulation of the EGF/EGFR signaling via E-cadherin-dependent manner in gastric cancer cells. PLoS One. 2013;8:e67542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 66. | Daşu A, Toma-Daşu I, Karlsson M. Theoretical simulation of tumour oxygenation and results from acute and chronic hypoxia. Phys Med Biol. 2003;48:2829-2842. [PubMed] [Cited in This Article: ] |
| 67. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510-5514. [PubMed] [Cited in This Article: ] |
| 68. | Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, Simon MC, Thompson CB. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037-1049. [PubMed] [Cited in This Article: ] |
| 69. | Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830-5835. [PubMed] [Cited in This Article: ] |
| 70. | Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411-421. [PubMed] [Cited in This Article: ] |
| 71. | Lin S, Ma R, Zheng XY, Yu H, Liang X, Lin H, Cai XJ. Meta-analysis of immunohistochemical expression of hypoxia inducible factor-1α as a prognostic role in gastric cancer. World J Gastroenterol. 2014;20:1107-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 19] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Graham EM, Edelsten C. Intermediate uveitis and sarcoidosis. Dev Ophthalmol. 1992;23:106-110. [PubMed] [Cited in This Article: ] |
| 73. | Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177-185. [PubMed] [Cited in This Article: ] |
| 74. | Chen Y, Gou X, Ke X, Cui H, Chen Z. Human tumor cells induce angiogenesis through positive feedback between CD147 and insulin-like growth factor-I. PLoS One. 2012;7:e40965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Zhang X, Yee D. Tyrosine kinase signalling in breast cancer: insulin-like growth factors and their receptors in breast cancer. Breast Cancer Res. 2000;2:170-175. [PubMed] [Cited in This Article: ] |
| 76. | Li H, Adachi Y, Yamamoto H, Min Y, Ohashi H, Ii M, Arimura Y, Endo T, Lee CT, Carbone DP. Insulin-like growth factor-I receptor blockade reduces tumor angiogenesis and enhances the effects of bevacizumab for a human gastric cancer cell line, MKN45. Cancer. 2011;117:3135-3147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 77. | Yuen JS, Macaulay VM. Targeting the type 1 insulin-like growth factor receptor as a treatment for cancer. Expert Opin Ther Targets. 2008;12:589-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 78. | Wang HB, Zhou CJ, Song SZ, Chen P, Xu WH, Liu B, Zhu KX, Yu WH, Wu HL, Wang HJ. Evaluation of Nrf2 and IGF-1 expression in benign, premalignant and malignant gastric lesions. Pathol Res Pract. 2011;207:169-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Li H, Xu L, Zhao L, Ma Y, Zhu Z, Liu Y, Qu X. Insulin-like growth factor-I induces epithelial to mesenchymal transition via GSK-3β and ZEB2 in the BGC-823 gastric cancer cell line. Oncol Lett. 2015;9:143-148. [PubMed] [Cited in This Article: ] |
| 80. | Min Y, Adachi Y, Yamamoto H, Imsumran A, Arimura Y, Endo T, Hinoda Y, Lee CT, Nadaf S, Carbone DP. Insulin-like growth factor I receptor blockade enhances chemotherapy and radiation responses and inhibits tumour growth in human gastric cancer xenografts. Gut. 2005;54:591-600. [PubMed] [Cited in This Article: ] |
| 81. | Gryko M, Kiśluk J, Cepowicz D, Zińczuk J, Kamocki Z, Guzińska-Ustymowicz K, Pryczynicz A, Czyżewska J, Kemona A, Kędra B. Expression of insulin-like growth factor receptor type 1 correlate with lymphatic metastases in human gastric cancer. Pol J Pathol. 2014;65:135-140. [PubMed] [Cited in This Article: ] |
| 82. | Tapia O, Riquelme I, Leal P, Sandoval A, Aedo S, Weber H, Letelier P, Bellolio E, Villaseca M, Garcia P. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014;465:25-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 83. | Liu JF, Zhou XK, Chen JH, Yi G, Chen HG, Ba MC, Lin SQ, Qi YC. Up-regulation of PIK3CA promotes metastasis in gastric carcinoma. World J Gastroenterol. 2010;16:4986-4991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 2014;4:64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 759] [Cited by in F6Publishing: 994] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 85. | Pedersen PL. Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr. 2007;39:211-222. [PubMed] [Cited in This Article: ] |
| 86. | Icard P, Lincet H. A global view of the biochemical pathways involved in the regulation of the metabolism of cancer cells. Biochim Biophys Acta. 2012;1826:423-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 87. | Smolková K, Plecitá-Hlavatá L, Bellance N, Benard G, Rossignol R, Ježek P. Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells. Int J Biochem Cell Biol. 2011;43:950-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 88. | Lutz MP, Zalcberg JR, Ducreux M, Ajani JA, Allum W, Aust D, Bang YJ, Cascinu S, Hölscher A, Jankowski J. Highlights of the EORTC St. Gallen International Expert Consensus on the primary therapy of gastric, gastroesophageal and oesophageal cancer - differential treatment strategies for subtypes of early gastroesophageal cancer. Eur J Cancer. 2012;48:2941-2953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 89. | Kim EY, Lee WJ, Choi D, Lee SJ, Choi JY, Kim BT, Kim HS. The value of PET/CT for preoperative staging of advanced gastric cancer: comparison with contrast-enhanced CT. Eur J Radiol. 2011;79:183-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 90. | Ma Q, Xin J, Zhao Z, Guo Q, Yu S, Xu W, Liu C, Zhai W. Value of 18F-FDG PET/CT in the diagnosis of primary gastric cancer via stomach distension. Eur J Radiol. 2013;82:e302-e306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Zhu Z, Li F, Mao Y, Cheng W, Cheng X, Dang Y. Improving evaluation of primary gastric malignancies by distending the stomach with milk immediately before 18F-FDG PET scanning. J Nucl Med Technol. 2008;36:25-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Lee JW, Lee SM, Lee MS, Shin HC. Role of 18F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging. 2012;39:1425-1434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 93. | Coupe NA, Karikios D, Chong S, Yap J, Ng W, Merrett N, Lin M. Metabolic information on staging FDG-PET-CT as a prognostic tool in the evaluation of 97 patients with gastric cancer. Ann Nucl Med. 2014;28:128-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Chung HW, Lee SY, Han HS, Park HS, Yang JH, Lee HH, So Y. Gastric cancers with microsatellite instability exhibit high fluorodeoxyglucose uptake on positron emission tomography. Gastric Cancer. 2013;16:185-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Liu P, Zhang XY, Shao Y, Zhang DF. Microsatellite instability in gastric cancer and pre-cancerous lesions. World J Gastroenterol. 2005;11:4904-4907. [PubMed] [Cited in This Article: ] |
| 96. | Pak KH, Yun M, Cheong JH, Hyung WJ, Choi SH, Noh SH. Clinical implication of FDG-PET in advanced gastric cancer with signet ring cell histology. J Surg Oncol. 2011;104:566-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 97. | Cayvarlı H, Bekiş R, Akman T, Altun D. The Role of 18F-FDG PET/CT in the Evaluation of Gastric Cancer Recurrence. Mol Imaging Radionucl Ther. 2014;23:76-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 98. | Tae CH, Lee JH, Choi JY, Min BH, Rhee PL, Kim JJ. Impact of incidental findings on integrated 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in patients with gastric cancer. Asia Pac J Clin Oncol. 2015;11:34-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 99. | D’Ugo D, Rausei S, Biondi A, Persiani R. Preoperative treatment and surgery in gastric cancer: friends or foes? Lancet Oncol. 2009;10:191-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Goerres GW, Stupp R, Barghouth G, Hany TF, Pestalozzi B, Dizendorf E, Schnyder P, Luthi F, von Schulthess GK, Leyvraz S. The value of PET, CT and in-line PET/CT in patients with gastrointestinal stromal tumours: long-term outcome of treatment with imatinib mesylate. Eur J Nucl Med Mol Imaging. 2005;32:153-162. [PubMed] [Cited in This Article: ] |
| 101. | Ott K, Herrmann K, Lordick F, Wieder H, Weber WA, Becker K, Buck AK, Dobritz M, Fink U, Ulm K. Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: long-term results of a prospective study. Clin Cancer Res. 2008;14:2012-2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 102. | Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28:247-253. [PubMed] [Cited in This Article: ] |
| 103. | Herrmann K, Ott K, Buck AK, Lordick F, Wilhelm D, Souvatzoglou M, Becker K, Schuster T, Wester HJ, Siewert JR. Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med. 2007;48:1945-1950. [PubMed] [Cited in This Article: ] |
| 104. | Lin Z, Mechalakos J, Nehmeh S, Schoder H, Lee N, Humm J, Ling CC. The influence of changes in tumor hypoxia on dose-painting treatment plans based on 18F-FMISO positron emission tomography. Int J Radiat Oncol Biol Phys. 2008;70:1219-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 105. | Krause BJ, Souvatzoglou M, Herrmann K, Weber AW, Schuster T, Buck AK, Nawroth R, Weirich G, Treiber U, Wester HJ. [11C]Choline as pharmacodynamic marker for therapy response assessment in a prostate cancer xenograft model. Eur J Nucl Med Mol Imaging. 2010;37:1861-1868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 106. | Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, Obradovich JE, Muzik O, Mangner TJ. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334-1336. [PubMed] [Cited in This Article: ] |
| 107. | Małkowski B, Staniuk T, Srutek E, Gorycki T, Zegarski W, Studniarek M. (18)F-FLT PET/CT in Patients with Gastric Carcinoma. Gastroenterol Res Pract. 2013;2013:696423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 108. | Zhou M, Wang C, Hu S, Zhang Y, Yao Z, Li J, Guo W, Zhang Y. 18F-FLT PET/CT imaging is not competent for the pretreatment evaluation of metastatic gastric cancer: a comparison with 18F-FDG PET/CT imaging. Nucl Med Commun. 2013;34:694-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 109. | Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134:714-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 556] [Cited by in F6Publishing: 549] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 110. | Jang M, Kim SS, Lee J. Cancer cell metabolism: implications for therapeutic targets. Exp Mol Med. 2013;45:e45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 111. | Arakaki AK, Skolnick J, McDonald JF. Marker metabolites can be therapeutic targets as well. Nature. 2008;456:443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 112. | Qiu Y, Cai G, Su M, Chen T, Liu Y, Xu Y, Ni Y, Zhao A, Cai S, Xu LX. Urinary metabonomic study on colorectal cancer. J Proteome Res. 2010;9:1627-1634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 113. | Gu H, Pan Z, Xi B, Asiago V, Musselman B, Raftery D. Principal component directed partial least squares analysis for combining nuclear magnetic resonance and mass spectrometry data in metabolomics: application to the detection of breast cancer. Anal Chim Acta. 2011;686:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 114. | Kim K, Aronov P, Zakharkin SO, Anderson D, Perroud B, Thompson IM, Weiss RH. Urine metabolomics analysis for kidney cancer detection and biomarker discovery. Mol Cell Proteomics. 2009;8:558-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 115. | Hori S, Nishiumi S, Kobayashi K, Shinohara M, Hatakeyama Y, Kotani Y, Hatano N, Maniwa Y, Nishio W, Bamba T. A metabolomic approach to lung cancer. Lung Cancer. 2011;74:284-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 116. | Kwon H, Oh S, Jin X, An YJ, Park S. Cancer metabolomics in basic science perspective. Arch Pharm Res. 2015;38:372-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 117. | Chan DC, Chen CJ, Chu HC, Chang WK, Yu JC, Chen YJ, Wen LL, Huang SC, Ku CH, Liu YC. Evaluation of serum amyloid A as a biomarker for gastric cancer. Ann Surg Oncol. 2007;14:84-93. [PubMed] [Cited in This Article: ] |
| 118. | Wang CS, Wu TL, Tsao KC, Sun CF. Serum TIMP-1 in gastric cancer patients: a potential prognostic biomarker. Ann Clin Lab Sci. 2006;36:23-30. [PubMed] [Cited in This Article: ] |
| 119. | Yu L, Aa J, Xu J, Sun M, Qian S, Cheng L, Yang S, Shi R. Metabolomic phenotype of gastric cancer and precancerous stages based on gas chromatography time-of-flight mass spectrometry. J Gastroenterol Hepatol. 2011;26:1290-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 120. | Abbassi-Ghadi N, Kumar S, Huang J, Goldin R, Takats Z, Hanna GB. Metabolomic profiling of oesophago-gastric cancer: a systematic review. Eur J Cancer. 2013;49:3625-3637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 121. | Chen JL, Tang HQ, Hu JD, Fan J, Hong J, Gu JZ. Metabolomics of gastric cancer metastasis detected by gas chromatography and mass spectrometry. World J Gastroenterol. 2010;16:5874-5880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 48] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 122. | Manerba M, Di Ianni L, Fiume L, Roberti M, Recanatini M, Di Stefano G. Lactate dehydrogenase inhibitors sensitize lymphoma cells to cisplatin without enhancing the drug effects on immortalized normal lymphocytes. Eur J Pharm Sci. 2015;74:95-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 123. | Bayet-Robert M, Loiseau D, Rio P, Demidem A, Barthomeuf C, Stepien G, Morvan D. Quantitative two-dimensional HRMAS 1H-NMR spectroscopy-based metabolite profiling of human cancer cell lines and response to chemotherapy. Magn Reson Med. 2010;63:1172-1183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 124. | Morvan D, Demidem A. Metabolomics by proton nuclear magnetic resonance spectroscopy of the response to chloroethylnitrosourea reveals drug efficacy and tumor adaptive metabolic pathways. Cancer Res. 2007;67:2150-2159. [PubMed] [Cited in This Article: ] |
| 125. | Wang X, Yan SK, Dai WX, Liu XR, Zhang WD, Wang JJ. A metabonomic approach to chemosensitivity prediction of cisplatin plus 5-fluorouracil in a human xenograft model of gastric cancer. Int J Cancer. 2010;127:2841-2850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 126. | Sasada S, Miyata Y, Tsutani Y, Tsuyama N, Masujima T, Hihara J, Okada M. Metabolomic analysis of dynamic response and drug resistance of gastric cancer cells to 5-fluorouracil. Oncol Rep. 2013;29:925-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 127. | Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R, Voelker HU, Thiede A, Coy JF. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer. 2008;8:122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 128. | Allen BG, Bhatia SK, Buatti JM, Brandt KE, Lindholm KE, Button AM, Szweda LI, Smith BJ, Spitz DR, Fath MA. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res. 2013;19:3905-3913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 129. | Wahdan-Alaswad R, Fan Z, Edgerton SM, Liu B, Deng XS, Arnadottir SS, Richer JK, Anderson SM, Thor AD. Glucose promotes breast cancer aggression and reduces metformin efficacy. Cell Cycle. 2013;12:3759-3769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 130. | Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804-10812. [PubMed] [Cited in This Article: ] |
| 131. | Chen J, Shi DY, Liu SL, Zhong L. Tanshinone IIA induces growth inhibition and apoptosis in gastric cancer in vitro and in vivo. Oncol Rep. 2012;27:523-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 132. | Lin LL, Hsia CR, Hsu CL, Huang HC, Juan HF. Integrating transcriptomics and proteomics to show that tanshinone IIA suppresses cell growth by blocking glucose metabolism in gastric cancer cells. BMC Genomics. 2015;16:41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 133. | Bhattacharya B, Low SH, Soh C, Kamal Mustapa N, Beloueche-Babari M, Koh KX, Loh J, Soong R. Increased drug resistance is associated with reduced glucose levels and an enhanced glycolysis phenotype. Br J Pharmacol. 2014;171:3255-3267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 134. | Granchi C, Fancelli D, Minutolo F. An update on therapeutic opportunities offered by cancer glycolytic metabolism. Bioorg Med Chem Lett. 2014;24:4915-4925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 135. | Ngo H, Tortorella SM, Ververis K, Karagiannis TC. The Warburg effect: molecular aspects and therapeutic possibilities. Mol Biol Rep. 2015;42:825-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 136. | Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol. 2009;19:17-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 425] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 137. | Maher JC, Krishan A, Lampidis TJ. Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol. 2004;53:116-122. [PubMed] [Cited in This Article: ] |
| 138. | Geschwind JF, Georgiades CS, Ko YH, Pedersen PL. Recently elucidated energy catabolism pathways provide opportunities for novel treatments in hepatocellular carcinoma. Expert Rev Anticancer Ther. 2004;4:449-457. [PubMed] [Cited in This Article: ] |
| 139. | Ganapathy-Kanniappan S, Geschwind JF, Kunjithapatham R, Buijs M, Vossen JA, Tchernyshyov I, Cole RN, Syed LH, Rao PP, Ota S. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell death. Anticancer Res. 2009;29:4909-4918. [PubMed] [Cited in This Article: ] |