Abstract
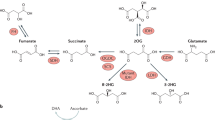
The phenomenon of enhanced glycolysis in tumours has been acknowledged for decades, but biochemical evidence to explain it is only just beginning to emerge. A significant hint as to the triggers and advantages of enhanced glycolysis in tumours was supplied by the recent discovery that succinate dehydrogenase (SDH) and fumarate hydratase (FH) are tumour suppressors and which associated, for the first time, mitochondrial enzymes and their dysfunction with tumorigenesis. Further steps forward showed that the substrates of SDH and FH, succinate and fumarate, respectively, can mediate a ‘metabolic signalling’ pathway. Succinate or fumarate, which accumulate in mitochondria owing to the inactivation of SDH or FH, leak out to the cytosol, where they inhibit a family of prolyl hydroxylase enzymes (PHDs). Depending on the PHD inhibited, two newly recognized pathways that support tumour maintenance may ensue: affected cells become resistant to certain apoptotic signals and/or activate a pseudohypoxic response that enhances glycolysis and is conveyed by hypoxia-inducible factor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y et al. (2002). Nat Genet 32: 614–621.
Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E et al. (2001). Am J Hum Genet 69: 49–54.
Baysal BE . (2003). Trends Endocrinol Metab 14: 453–459.
Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A et al. (2000). Science 287: 848–851.
Baysal BE, Willett-Brozick JE, Filho PA, Lawrence EC, Myers EN, Ferrell RE . (2004). J Med Genet 41: 703–709.
Benn DE . (2006). J Clin Endorcrinol Metab 91: 827–836.
Briere JJ, Favier J, Benit P, El Ghouzzi V, Lorenzato A, Rabier D et al. (2005). Hum Mol Genet 14: 3263–3269.
Carew JS, Huang P . (2002). Mol Cancer 1: 9.
Chau NM, Rogers P, Aherne W, Carroll V, Collins I, McDonald E et al. (2005). Cancer Res 65: 4918–4928.
Dahia PL . (2006). Curr Opin Oncol 18: 1–8.
Dahia PLM, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M et al. (2005). PLoS Geneti 1: e8.
Dang CV, Semenza GL . (1999). Trends Biochem Sci 24: 68–72.
Dann III CE, Bruick RK . (2005). Biochem Biophys Res Commun 338: 639–647.
Douwes Dekker PB, Hogendoorn PC, Kuipers-Dijkshoorn N, Prins FA, van Duinen SG, Taschner PE et al. (2003). J Pathol 201: 480–486.
Elson DA, Thurston G, Huang LE, Ginzinger DG, McDonald DM, Johnson RS et al. (2001). Genes Dev 15: 2520–2532.
Eng C, Kiuru M, Fernandez MJ, Aaltonen LA . (2003). Nat Rev Cancer 3: 193–202.
Favier J, Briere JJ, Strompf L, Amar L, Filali M, Jeunemaitre X et al. (2005). Horm Res 63: 171–179.
Gatenby RA, Gillies RJ . (2004). Nat Rev Cancer 4: 891–899.
Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D et al. (2004). Cell 118: 781–794.
Gimenez-Roqueplo AP, Favier J, Rustin P, Mourad JJ, Plouin PF, Corvol P et al. (2001). Am J Hum Genet 69: 1186–1197.
Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Kerlan V, Plouin PF et al. (2002). J Clin Endocrinol Metab 87: 4771–4774.
Gottlieb E, Tomlinson IP . (2005). Nat Rev Cancer 5: 857–866.
Green DR, Kroemer G . (2005). J Clin Invest 115: 2610–2617.
Hammerman PS, Fox CJ, Thompson CB . (2004). Trends Biochem Sci 29: 586–592.
Hewitson KS, Schofield CJ . (2004). Drug Discov Today 9: 704–711.
Hoffman MA, Ohh M, Yang H, Klco JM, Ivan M, Kaelin Jr WG . (2001). Hum Mol Genet 10: 1019–1027.
Inoki K, Corradetti MN, Guan KL . (2005). Nat Genet 37: 19–24.
Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL et al. (2005). Cancer Cell 8: 143–153.
Ishii N, Fujii M, Hartman PS, Tsuda M, Yasuda K, Senoo-Matsuda N et al. (1998). Nature 394: 694–697.
Ishii T, Yasuda K, Akatsuka A, Hino O, Hartman PS, Ishii N . (2005). Cancer Res 65: 203–209.
Kim WY, Kaelin WG . (2004). J Clin Oncol 22: 4991–5004.
Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A et al. (2004). Cancer Cell 6: 33–43.
Lee S, Nakamura E, Yang H, Wei W, Linggi MS, Sajan MP et al. (2005). Cancer Cell 8: 155–167.
Lehtonen R, Kiuru M, Vanharanta S, Sjoberg J, Aaltonen LM, Aittomaki K et al. (2004). Am J Pathol 164: 17–22.
Maxwell PH . (2005a). Semin Cell Dev Biol 16: 523–530.
Maxwell PH . (2005b). Exp Physiol 90: 791–797.
Modica-Napolitano JS, Singh KK . (2004). Mitochondrion 4: 755–762.
Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, Muresan M et al. (2004). JAMA 292: 943–951.
Niemann S, Muller U . (2000). Nat Genet 26: 268–270.
Owen OE, Kalhan SC, Hanson RW . (2002). J Biol Chem 277: 30409–30412.
Pawlu C, Bausch B, Neumann HP . (2005). Fam Cancer 4: 49–54.
Percy MJ . (2006). Proc Natl Acad Sci USA 103: 654–659.
Plas DR, Thompson CB . (2005). Oncogene 24: 7435–7442.
Pollard P, Wortham N, Barclay E, Alam A, Elia G, Manek S et al. (2005a). J Pathol 205: 41–49.
Pollard PJ, Briere JJ, Alam NA, Barwell J, Barclay E, Wortham NC et al. (2005b). Hum Mol Genet 14: 2231–2239.
Raha S, Robinson BH . (2000). Trends Biochem Sci 25: 502–508.
Robey RB, Hay N . (2005). Cell Cycle 4: 654–658.
Rustin P, Rotig A . (2002). Biochim Biophys Acta 1553: 117–122.
Schiavi F, Boedeker CC, Bausch B, Peczkowska M, Gomez CF, Strassburg T et al. (2005). JAMA 294: 2057–2063.
Schofield CJ, Ratcliffe PJ . (2004). Nat Rev Mol Cell Biol 5: 343–354.
Schofield CJ, Ratcliffe PJ . (2005). Biochem Biophys Res Commun 338: 617–626.
Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD et al. (2005). Cancer Cell 7: 77–85.
Senoo-Matsuda N, Yasuda K, Tsuda M, Ohkubo T, Yoshimura S, Nakazawa H et al. (2001). J Biol Chem 276: 41553–41558.
Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D et al. (2002). Nat Genet 30: 406–410.
Vanharanta S, Buchta M, McWhinney SR, Virta SK, Peczkowska M, Morrison CD et al. (2004). Am J Hum Genet 74: 153–159.
Vanharanta S, Pollard PJ, Lehtonen HJ, Laiho P, Sjoberg J, Leminen A et al. (2006). Hum Mol Genet 15: 97–103.
Wei MH, Toure O, Glenn GM, Pithukpakorn M, Neckers L, Stolle C et al. (2006). J Med Genet 43: 18–27.
Welsh S, Williams R, Kirkpatrick L, Paine-Murrieta G, Powis G . (2004). Mol Cancer Ther 3: 233–244.
Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C et al. (2003). Science 299: 700–704.
Zu XL, Guppy M . (2004). Biochem Biophys Res Commun 313: 459–465.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
King, A., Selak, M. & Gottlieb, E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene 25, 4675–4682 (2006). https://doi.org/10.1038/sj.onc.1209594
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209594
Keywords
This article is cited by
-
Sarcosine (glycine transporter inhibitor) attenuates behavioural and biochemical changes induced by ketamine, in the rat model of schizophrenia
Experimental Brain Research (2023)
-
Oncogenic metabolic reprogramming in breast cancer: focus on signaling pathways and mitochondrial genes
Medical Oncology (2023)
-
MicroRNAs as the critical regulators of cell migration and invasion in thyroid cancer
Biomarker Research (2022)
-
Cancer-derived extracellular succinate: a driver of cancer metastasis
Journal of Biomedical Science (2022)
-
Serum metabolomic profiling for patients with adenocarcinoma of the esophagogastric junction
Metabolomics (2022)