Abstract
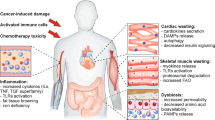
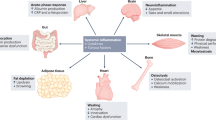
Cachexia is a systemic condition that occurs during many neoplastic diseases, such as cancer. Cachexia in cancer is characterized by loss of body weight and muscle and by adipose tissue wasting and systemic inflammation. Cancer cachexia is often associated with anorexia and increased energy expenditure. Even though the cachectic condition severely affects skeletal muscle, a tissue that accounts for ~40% of total body weight, it represents a multi-organ syndrome that involves tissues and organs such as white adipose tissue, brown adipose tissue, bone, brain, liver, gut and heart. Indeed, evidence suggests that non-muscle tissues and organs, as well as tumour tissues, secrete soluble factors that act on skeletal muscle to promote wasting. In addition, muscle tissue also releases various factors that can interact with the metabolism of other tissues during cancer. In this Review, we examine the effect of non-muscle tissues and inter-tissue communication in cancer cachexia and discuss studies aimed at developing novel therapeutic strategies for the condition.
Key points
-
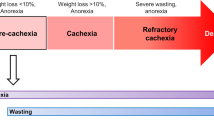
Cancer cachexia is an energy-wasting syndrome caused by decreased food intake (owing to marked anorexia) and increased energy expenditure.
-
Cancer cachexia affects many tissues, including adipose tissues, heart, bone, liver, gastrointestinal tract and brain.
-
Systemic inflammation is a hallmark of patients with cancer, and the inflammatory response is involved in the metabolic alterations present during cancer cachexia.
-
The balance between pro-cachectic cytokines and anti-cachectic cytokines, both of which are produced by many cell types, might actually determine the cachectic response.
-
The importance of the metabolic changes that occurr in white and brown adipose tissues during cancer cachexia have been underestimated.
-
We need to unravel the communication between skeletal muscle and other tissues to better understand the aetiology of cancer cachexia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Evans, W. J. et al. Cachexia: a new definition. Clin. Nutr. 27, 793–799 (2008).
Argilés, J. M., Busquets, S., Stemmler, B. & López-Soriano, F. J. Cancer cachexia: understanding the molecular basis. Nat. Rev. Cancer 14, 754–762 (2014).
Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 (2011).
Stewart, G. D., Skipworth, R. J. & Fearon, K. C. Cancer cachexia and fatigue. Clin. Med. 6, 140–143 (2006).
Warren, S. The immediate cause of death in cancer. Am. J. Med. Sci. 184, 610–613 (1932).
Coletti, D. Chemotherapy-induced muscle wasting: an update. Eur. J. Transl Myol. 28, 7587 (2018).
Dewys, W. D. et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am. J. Med. 69, 491–497 (1980).
Arthur, S. T. et al. Cachexia among US cancer patients. J. Med. Econ. 19, 874–880 (2016).
Argilés, J. M. The 2015 ESPEN Sir David Cuthbertson lecture: Inflammation as the driving force of muscle wasting in cancer. Clin. Nutr. 36, 798–803 (2016).
Argilés, J. M., López-Soriano, F. J. & Busquets, S. Counteracting inflammation: a promising therapy in cachexia. Crit. Rev. Oncog. 17, 253–262 (2012).
Bonetto, A. et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 303, E410–E421 (2012).
Camargo, R. G. et al. NF-κBp65 and expression of its pro-inflammatory target genes are upregulated in the subcutaneous adipose tissue of cachectic cancer patients. Nutrients 7, 4465–4479 (2015).
Waning, D. L. & Guise, T. A. Molecular Mechanisms of Bone Metastasis and Associated Muscle Weakness. Clin. Cancer Res. 20, 3071–3077 (2014)
Tisdale, M. J. Are tumoral factors responsible for host tissue wasting in cancer cachexia? Future Oncol. 6, 503–513 (2010).
Bing, C., Mracek, T., Gao, D. & Trayhurn, P. Zinc-2-glycoprotein: an adipokine modulator of body fat mass? Int. J. Obes. 34, 1559–1965 (2010).
Argilés, J. M., Orpí, M., Busquets, S. & López-Soriano, F. J. Myostatin: more than just a regulator of muscle mass. Drug Discov. Today. 17, 702–709 (2012).
Zhou, X. et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142, 531–543 (2010).
Bossola, M., Marzetti, E., Rosa, F. & Pacelli, F. Skeletal muscle regeneration in cancer cachexia. Clin. Exp. Pharmacol. Physiol. 43, 522–527 (2016).
Sandri, M. Protein breakdown in cancer cachexia. Semin. Cell Dev. Biol. 54, 11–19 (2016).
Cohen, S., Zhai, B., Gygi, S. P. & Goldberg, A. L. Ubiquitylation by Trim32 causes coupled loss of desmin, Z-bands, and thin filaments in muscle atrophy. J. Cell Biol. 198, 575–589 (2012).
Costelli, P. et al. Ca2+-dependent proteolysis in muscle wasting. Int. J. Biochem. Cell Biol. 37, 2134–2146 (2005).
Jain, S., Gautam, V. & Naseem, S. Acute-phase proteins: as diagnostic tool. J. Pharm. Bioallied Sci. 3, 118–127 (2011).
Andersson, C., Gelin, J., Iresjö, B. M. & Lundholm, K. Acute-phase proteins in response to tumor growth. J. Surg. Res. 55, 607–614 (1993).
Richards, C. H. et al. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLOS ONE 7, e41883 (2012).
Roxburgh, C. S. & McMillan, D. C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Futur. Oncol. 6, 149–163 (2010).
Proctor, M. J. et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br. J. Cancer 104, 726–734 (2011).
Simmons, C. P. L. et al. Prognostic tools in patients with advanced cancer: a systematic review. J. Pain Symptom Manage. 53, 962–970.e10 (2017).
Zhang, L. et al. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J. Am. Soc. Nephrol. 20, 604–612 (2009).
Argilés, J. M., Alvarez, B. & López-Soriano, F. J. The metabolic basis of cancer cachexia. Med. Res. Rev. 17, 477–498 (1997).
Prokopchuk, O. et al. IL-4 mRNA is downregulated in the liver of pancreatic cancer patients suffering from cachexia. Nutr. Cancer 69, 84–91 (2017).
Peyta, L. et al. Regulation of hepatic cardiolipin metabolism by TNF-α: implication in cancer cachexia. Biochim. Biophys. Acta 1851, 1490–1500 (2015).
Dudek, J. Role of cardiolipin in mitochondrial signaling pathways. Front. Cell Dev. Biol. 75, 90 (2017).
Di Gregorio, G. B. et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes 54, 2305–2313 (2005).
Martignoni, M. E. et al. Liver macrophages contribute to pancreatic cancer-related cachexia. Oncol. Rep. 21, 363–369 (2009).
Klein, G. L., Petschow, B. W., Shaw, A. L. & Weaver, E. Gut barrier dysfunction and microbial translocation in cancer cachexia: a new therapeutic target. Curr. Opin. Support Palliat. Care 7, 361–367 (2013).
Puppa, M. J. et al. Gut barrier dysfunction in the Apc(Min/+) mouse model of colon cancer cachexia. Biochim. Biophys. Acta 1812, 1601–1606 (2011).
Kufe, D. W. Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer 9, 874–885 (2009).
Matsuura, M. Structural modifications of bacterial lipopolysaccharide that facilitate Gram-negative bacteria evasion of host innate immunity. Front. Immunol. 4, 109 (2013).
Soler, A. P. et al. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis 20, 1425–1431 (1999).
Ohtani, S. et al. Expression of tight-junction-associated proteins in human gastric cancer: downregulation of claudin-4 correlates with tumor aggressiveness and survival. Gastr. Cancer 12, 43–51 (2009).
Bindels, L. B. et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLOS ONE 7, e37971 (2012).
Bindels, L. B. et al. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J. 10, 1456–1470 (2016).
Bindels, L. B. & Delzenne, N. M. Muscle wasting: the gut microbiota as a new therapeutic target? Int. J. Biochem. Cell Biol. 45, 2186–2190 (2013).
Watanabe, M. et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489 (2006).
Manasa, S. et al. Role of the normal gut microbiota. World J. Gastroenterol. 21, 8787–8803 (2015).
Sakuma, K., Aoi, W. & Yamaguchi, A. Molecular mechanism of sarcopenia and cachexia: recent research advances. Pflügers Arch. 469, 573–591 (2017).
Bäckhed, F., Manchester, J. K., Semenkovich, C. F. & Gordon, J. I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl Acad. Sci. USA 104, 979–984 (2007).
Delzenne, N. M., Neyrinck, A. M., Bäckhed, F. & Cani, P. D. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 7, 639–646 (2011).
Sandri, M. et al. PGC-1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl Acad. Sci. USA 103, 16260–16265 (2006).
Kojima, M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 (1999).
Castañeda, T. R., Tong, J., Datta, R., Culler, M. & Tschöp, M. H. Ghrelin in the regulation of body weight and metabolism. Front. Neuroendocr. 31, 44–60 (2010).
Sousa-Ferreira, L. et al. Proliferative hypothalamic neurospheres express NPY, AGRP, POMC, CART and Orexin-A and differentiate to functional neurons. PLOS ONE 6, e19745 (2011).
Choi, K. et al. The role of ghrelin and growth hormone secretagogues receptor on rat adipogenesis. Endocrinology 144, 754–759 (2003).
Mano-Otagiri, A. et al. Genetic suppression of ghrelin receptors activates brown adipocyte function and decreases fat storage in rats. Regul. Pept. 160, 81–90 (2010).
Granado, M., Martin, A. I., Villanua, M. A. & Lopez-Calderon, A. Experimental arthritis inhibits the insulin-like growth factor-I axis and induces muscle wasting through cyclooxygenase-2 activation. Am. J. Physiol. Endocrinol. Metab. 292, 1656–1665 (2007).
Zeng, X., Chen, S., Yang, Y. & Ke, Z. Acylated and unacylated ghrelin inhibit atrophy in myotubes co-cultured with colon carcinoma cells. Oncotarget 8, 72872–72885 (2017).
Zeng, X., Chen, S., Lin, Y. & Ke, Z. Acylated and unacylated ghrelin inhibit apoptosis in myoblasts cocultured with colon carcinoma cells. Oncol. Rep. 39, 1387–1395 (2018).
Wang, H. S., Oh, D. S., Ohning, G. V. & Pisegna, J. R. Elevated serum ghrelin exerts an orexigenic effect that may maintain body mass index in patients with metastatic neuroendocrine tumors. J. Mol. Neurosci. 33, 225–231 (2007).
Kerem, M. et al. Adipokines and ghrelin in gastric cancer cachexia. World J. Gastroenterol. 14, 3633–3641 (2008).
Takahashi, M. et al. Ghrelin and leptin levels in cachectic patients with cancer of the digestive organs. Int. J. Clin. Oncol. 14, 315–320 (2009).
Karapanagiotou, E. M. et al. Increased serum levels of ghrelin at diagnosis mediate body weight loss in non-small cell lung cancer (NSCLC) patients. Lung Cancer 66, 393–398 (2009).
Terawaki, K. et al. Development of ghrelin resistance in a cancer cachexia rat model using human gastric cancer-derived 85As2 cells and the palliative effects of the Kampo medicine rikkunshito on the model. PLOS ONE 12, e0173113 (2017).
Harmatz, E. S. et al. Central ghrelin resistance permits the overconsolidation of fear memory. Biol. Psychiatry. 81, 1003–1013 (2017).
Katakami, N. et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 124, 606–616 (2018).
Bai, Y. et al. Anamorelin for cancer anorexia-cachexia syndrome: a systematic review and meta-analysis. Support. Care Cancer 25, 1651–1659 (2017).
Graf, S. A. & Garcia, J. M. Anamorelin hydrochloride in the treatment of cancer anorexia-cachexia syndrome: design, development, and potential place in therapy. Drug Des. Devel. Ther. 11, 2325–2331 (2017).
Majchrzak, K., Szyszko, K., Pawłowski, K. M., Motyl, T. & Król, M. A role of ghrelin in cancerogenesis. Pol. J. Vet. Sci. 15, 189–197 (2012).
Costa, J. L. et al. Ghrelin is an osteoblast mitogen and increases osteoclastic bone resorption in vitro. Int. J. Pept. 2011, 605193 (2011).
Giralt, M., Cereijo, R. & Villarroya, F. Adipokines and the endocrine role of adipose tissues. Handb. Exp. Pharmacol. 233, 265–282 (2016).
Vaitkus, J. A. & Celi, F. S. The role of adipose tissue in cancer-associated cachexia. Exp. Biol. Med. 242, 473–481 (2017).
Dong, M., Lin, J., Lim, W., Jin, W. & Lee, H. J. Role of brown adipose tissue in metabolic syndrome, aging, and cancer cachexia. Front. Med. 12, 130–138 (2018).
Dahlman, I. et al. Adipose tissue pathways involved in weight loss of cancer cachexia. Br. J. Cancer 102, 1541–1548 (2010).
Silvério, R. et al. Lipases and lipid droplet-associated protein expression in subcutaneous white adipose tissue of cachectic patients with cancer. Lipids Health Dis. 16, 159 (2017).
Contreras, G. A., Strieder-Barboza, C. & Raphael, W. Adipose tissue lipolysis and remodeling during the transition period of dairy cows. J. Anim. Sci. Biotechnol. 8, 41 (2017).
Batista, M. L. et al. Adipose tissue inflammation and cancer cachexia: possible role of nuclear transcription factors. Cytokine 57, 9–16 (2012).
Argilés, J. M., López-Soriano, F. J. & Busquets, S. Mechanisms to explain wasting of muscle and fat in cancer cachexia. Curr. Opin. Support. Palliat. Care 1, 293–298 (2008).
Han, J., Meng, Q., Shen, L. & Wu, G. Interleukin-6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 17, 14 (2018).
Mracek, T. et al. Enhanced ZAG production by subcutaneous adipose tissue is linked to weight loss in gastrointestinal cancer patients. Br. J. Cancer 104, 441–447 (2011).
Geer, E. B., Islam, J. & Buettner, C. Mechanisms of glucocorticoid-induced insulin resistance focus on adipose tissue function and lipid metabolism. Endocrinol. Metab. Clin. North Am. 43, 75–102 (2014).
Abdullahi, A. & Jeschke, M. G. Taming the flames: targeting white adipose tissue browning in hypermetabolic conditions. Endocr. Rev. 38, 538–549 (2017).
Kir, S. & Spiegelman, B. M. Cachexia and brown fat: a burning issue in cancer. Trends Cancer 2, 461–463 (2016).
Petruzzelli, M. et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447 (2014).
Rohm, M. et al. An AMP-activated protein kinase-stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Nat. Med. 22, 1120–1130 (2016).
Tsoli, M. et al. Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Cancer Res. 72, 4372–4382 (2012).
Basu, S. Hypothesizing association between cancer cachexia and Fluorodeoxyglucose-positron emission tomography documented brown adipose tissue hypermetabolism in cancer patients with an illustration in grossly emaciated cachectic patient in hot Indian summer climate: will beta blockers find use in the management of this condition? Indian J. Cancer 52, 223 (2015).
Argilés, J. M., López-Soriano, J., Almendro, V., Busquets, S. & López-Soriano, F. J. Cross-talk between skeletal muscle and adipose tissue: a link with obesity? Med. Res. Rev. 25, 49–65 (2005).
Makki, K., Froguel, P. & Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013, 139239 (2013).
Argilés, J. M., López-Soriano, F. J. & Busquets, S. Therapeutic potential of interleukin-15: a myokine involved in muscle wasting and adiposity. Drug Discov. Today 14, 208–213 (2009).
Das, S. K. et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 333, 233–238 (2011).
Wang, S. & Yang, X. Inter-organ regulation of adipose tissue browning. Cell. Mol. Life Sci. 74, 1765–1776 (2017).
Moreno-Navarrete, J. M. et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 98, E769–E778 (2013).
Boström, P. et al. A PGC1-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012).
Shan, T., Liang, X., Bi, P. & Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1-Fndc5 pathway in muscle. FASEB J. 27, 1981–1989 (2013).
Carriere, A. et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63, 3253–3265 (2014).
Stephens, N. A. et al. Intramyocellular lipid droplets increase with progression of cachexia in cancer patients. J. Cachexia Sarcopenia Muscle 2, 111–117 (2011).
Ambrus, J. L., Ambrus, C. M., Mink, I. B. & Pickren, J. W. Causes of death in cancer patients. J. Med. 6, 61–64 (1975).
Dudgeon, D. & Baracos, V. E. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr. Opin.Support. Palliat. Care 10, 236–241 (2016).
Drott, C. & Lundholm, K. Glucose uptake and amino acid metabolism in perfused hearts from tumor-bearing rats. J. Surg. Res. 49, 62–68 (1990).
Hyltander, A., Drott, C., Körner, U., Sandström, R. & Lundholm, K. Elevated energy expenditure in cancer patients with solid tumours. Eur. J. Cancer 27, 9–15 (1991).
Barkhudaryan, A., Scherbakov, N., Springer, J. & Doehner, W. Cardiac muscle wasting in individuals with cancer cachexia. ESC Heart Fail. 4, 458–467 (2017).
Olivan, M. et al. Theophylline is able to partially revert cachexia in tumour-bearing rats. Nutr. Metab. 9, 76 (2012).
Shirazi, L. F., Bissett, J., Romeo, F. & Mehta, J. L. Role of inflammation in heart failure. Curr. Atheroscler. Rep. 19, 27 (2017).
Belloum, Y., Rannou-Bekono, F. & Favier, F. B. Cancer-induced cardiac cachexia: pathogenesis and impact of physical activity (Review). Oncol. Rep. 37, 2543–2552 (2017).
Reuter, S., Gupta, S. C., Chaturvedi, M. M. & Aggarwal, B. B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 49, 1603–1616 (2010).
Tian, M. et al. Cardiac alterations in cancer-induced cachexia in mice. Int. J. Oncol. 37, 347–353 (2010).
Mühlfeld, C. et al. Cancer induces cardiomyocyte remodeling and hypoinnervation in the left ventricle of the mouse heart. PLOS ONE 6, e20424 (2011).
Cosper, P. F. & Leinwand, L. A. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Res. 71, 1710–1720 (2011).
Zimmers, T. A. et al. Exogenous GDF11 induces cardiac and skeletal muscle dysfunction and wasting. Basic Res. Cardiol. 112, 48 (2017).
Wysong, A. et al. NF-B inhibition protects against tumor-induced cardiac atrophy in vivo. Am. J. Pathol. 178, 1059–1068 (2011).
Burjonroppa, S. C. et al. Cancer patients with markedly elevated B-type natriuretic peptide may not have volume overload. Am. J. Clin. Oncol. 30, 287–293 (2007).
Ritchie, R. H., Rosenkranz, A. C. & Kaye, D. M. B-Type natriuretic peptide: endogenous regulator of myocardial structure, biomarker and therapeutic target. Curr. Mol. Med. 9, 814–825 (2009).
Tian, M., Asp, M. L., Nishijima, Y. & Belury, M. A. Evidence for cardiac atrophic remodeling in cancer-induced cachexia in mice. Int. J. Oncol. 39, 1321–1326 (2011).
Ogawa, T. & de Bold, A. J. The heart as an endocrine organ. Endocr. Connect. 3, R31–R44 (2014).
Delafontaine, P. & Yoshida, T. The renin-angiotensin system and the biology of skeletal muscle: mechanisms of muscle wasting in chronic disease states. Trans. Am. Clin. Climatol. Assoc. 127, 245–258 (2016).
Penafuerte, C. A. et al. Identification of neutrophil-derived proteases and angiotensin II as biomarkers of cancer cachexia. Br. J. Cancer 114, 680–687 (2016).
Kurtz, A., Della Bruna, R., Pfeilschifter, J., Taugner, R. & Bauer, C. Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP-mediated process. Proc. Natl Acad. Sci. USA 83, 4769–4773 (1986).
Gruden, G., Landi, A. & Bruno G. Natriuretic peptides, heart, and adipose tissue: new findings and future developments for diabetes research. Diabetes Care. 37, 2899–2908 (2014).
Nojiri, T. et al. Atrial natriuretic peptide prevents cancer metastasis through vascular endothelial cells. Proc. Natl Acad. Sci. USA 112, 4086–4091 (2015).
Solheim, T. S. et al. Is there a genetic cause of appetite loss? — an explorative study in 1,853 cancer patients. J. Cachexia Sarcopenia Muscle 3, 191–198 (2012).
van Norren, K., Dwarkasing, J. T. & Witkamp, R. F. The role of hypothalamic inflammation, the hypothalamic–pituitary–adrenal axis and serotonin in the cancer anorexia–cachexia syndrome. Curr. Opin. Clin. Nutr. Metab. Care 20, 396–401 (2017).
Cai, D. & Liu, T. Hypothalamic inflammation: a double-edged sword to nutritional diseases. Ann. NY Acad. Sci. 1243, E1–E39 (2011).
Burfeind, K. G., Michaelis, K. A. & Marksa, D. L. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin. Cell Dev. Biol. 54, 42–52 (2016).
Dwarkasing, J. T. et al. Differences in food intake of tumour-bearing cachectic mice are associated with hypothalamic serotonin signalling. J. Cachexia Sarcopenia Muscle 6, 84–94 (2015).
Dwarkasing, J. T. et al. Increased hypothalamic serotonin turnover in inflammation-induced anorexia. BMC Neurosci. 17, 26 (2016).
Molfino, A. et al. Cancer anorexia: hypothalamic activity and its association with inflammation and appetite-regulating peptides in lung cancer. J. Cachexia Sarcopenia Muscle 8, 40–47 (2017).
Evans, W. K. et al. Limited impact of total parenteral nutrition on nutritional status during treatment for small cell lung cancer. Cancer Res. 45, 3347–3353 (1985).
Bodine, S. C. & Furlow, J. D. Glucocorticoids and skeletal muscle. Adv. Exp. Med. Biol. 872, 145–176 (2015).
Joppa, M. A., Gogas, K. R., Foster, A. C. & Markison, S. Central infusion of the melanocortin receptor antagonist agouti-related peptide (AgRP(83–132)) prevents cachexia-related symptoms induced by radiation and colon-26 tumors in mice. Peptides 28, 636–642 (2007).
Cheung, W. W. & Mak, R. H. Melanocortin antagonism ameliorates muscle wasting and inflammation in chronic kidney disease. Am. J. Physiol. Renal Physiol. 303, F1315–F1324 (2012).
Murtaza, B. et al. Alteration in taste perception in cancer: causes and strategies of treatment. Front. Physiol. 8, 134 (2017).
Tohgo, A., Kumazawa, E., Akahane, K., Asakawa, A. & Inui, A. Anticancer drugs that induce cancer-associated cachectic syndromes. Expert Rev. Anticancer Ther. 2, 121–129 (2002).
Hopkinson, J. B. The emotional aspects of cancer anorexia. Curr. Opin. Support. Palliat. Care 4, 254–258 (2010).
Lerner, L. et al. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J. Cachexia Sarcopenia Muscle 6, 317–324 (2015).
Breit, S. N., Tsai, V. W.-W. & Brown, D. A. Targeting obesity and cachexia: identification of the GFRAL receptor–MIC-1/GDF15 pathway. Trends Mol. Med. 23, 1065–1067 (2017).
Borner, T., Liberini, C. G., Lutz, T. A. & Riediger, T. Brainstem GLP-1 signalling contributes to cancer anorexia-cachexia syndrome in the rat. Neuropharmacology 131, 282–290 (2018).
Meloni, A. R. et al. GLP-1 receptor activated insulin secretion from pancreatic-cells: mechanism and glucose dependence. Diabetes Obes. Metab. 15, 15–27 (2013).
Grossberg, A. J., Scarlett, J. M. & Marks, D. L. Hypothalamic mechanisms in cachexia. Physiol. Behav. 100, 478–489 (2010).
Molfino, A., Laviano, A. & Rossi Fanelli, F. Contribution of anorexia to tissue wasting in cachexia. Curr. Opin.Support. Palliat. Care 4, 249–253 (2010).
Bonetto, A. et al. Differential bone loss in mouse models of colon cancer cachexia. Front. Physiol. 7, 679 (2017).
Regan, J. N., Trivedi, T., Guise, T. A. & Waning, D. L. The role of TGF in bone-muscle crosstalk. Curr. Osteoporos. Rep. 15, 18–23 (2017).
Sartori, R. et al. Smad2 and 3 transcription factors control muscle mass in adulthood. Am. J. Physiol. Cell Physiol. 296, C1248–C1257 (2009).
Chambard, L. et al. Bone, muscle, and metabolic parameters predict survival in patients with synchronous bone metastases from lung cancers. Bone 108, 202–209 (2018).
Regan, J. N. et al. Osteolytic breast cancer causes skeletal muscle weakness in an immunocompetent syngeneic mouse model. Front. Endocrinol. 8, 358 (2017).
Meissner, G. The structural basis of ryanodine receptor ion channel function. J. Gen. Physiol. 149, 1065–1089 (2017).
Stammers, A. N. et al. The regulation of sarco(endo)plasmic reticulum calcium-ATPases (SERCA). Can. J. Physiol. Pharmacol. 93, 1–12 (2015).
Laurent, M. R. et al. Muscle-bone interactions: From experimental models to the clinic? A critical update. Mol. Cell. Endocrinol. 432, 14–36 (2016).
Quinn, L. S., Anderson, B. G., Strait-Bodey, L., Stroud, A. M. & Argilés, J. M. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am. J. Physiol. Metab. 296, E191–E202 (2009).
Martínez-Hernández, P. L. et al. Serum interleukin-15 levels in cancer patients with cachexia. Oncol. Rep. 28, 1443–1452 (2012).
Argiles, J. M., López-Soriano, F. J., Stemmler, B. & Busquets, S. Novel targeted therapies for cancer cachexia. Biochem. J. 474, 2663–2678 (2017).
Gullett, N. P. et al. Nutritional interventions for cancer-induced cachexia. Curr. Probl. Cancer 35, 58–90 (2011).
Delzenne, N. M. & Bindels, L. B. Gut microbiota in 2017: contribution of gut microbiota–host cooperation to drug efficacy. Nat. Rev. Gastroenterol. Hepatol. 15, 69–70 (2017).
Shrotriya, S., Walsh, D., Bennani-Baiti, N., Thomas, S. & Lorton, C. C-Reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic review. PLOS ONE 10, e0143080 (2015).
Argilés, J. M. et al. Validation of the CAchexia SCOre (CASCO). Staging cancer patients: the use of minicasco as a simplified tool. Front. Physiol. 8, 92 (2017).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
Argilés, J. M. et al. Cachexia: a problem of energetic inefficiency. J. Cachexia Sarcopenia Muscle 5, 279–286 (2014).
Del Fabbro, E., Orr, T. A. & Stella, S. M. Practical approaches to managing cancer patients with weight loss. Curr. Opin. Support. Palliat. Care 11, 272–277 (2017).
Valassi, E., Scacchi, M. & Cavagnini, F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 18, 158–168 (2008).
Lau, J. & Herzog, H. CART in the regulation of appetite and energy homeostasis. Front. Neurosci. 8, 313 (2014).
Crespo, C. S. et al. Peptides and food intake. Front. Endocrinol. 5, 58 (2014).
Tecott, L. H. Serotonin and the orchestration of energy balance. Cell. Metabolism 6, 352–361 (2007).
Davos, C. H. et al. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J. Card. Fail. 9, 29–35 (2003).
Acknowledgements
The work of the authors is supported by a grant from the Ministerio de Ciencia y Tecnología (MCyT) (SAF2015-65589-P).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
B.S. is an employee of BSA Nutrition Center, which is a non-academic, for-profit affiliation. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Argilés, J.M., Stemmler, B., López-Soriano, F.J. et al. Inter-tissue communication in cancer cachexia. Nat Rev Endocrinol 15, 9–20 (2019). https://doi.org/10.1038/s41574-018-0123-0
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-018-0123-0
This article is cited by
-
Effect of preoperative immunonutrition on postoperative short-term clinical outcomes in patients with gastric cancer cachexia: a prospective randomized controlled trial
World Journal of Surgical Oncology (2024)
-
Leukemia inhibitory factor suppresses hepatic de novo lipogenesis and induces cachexia in mice
Nature Communications (2024)
-
Virtual reality-empowered deep-learning analysis of brain cells
Nature Methods (2024)
-
Cancer-triggered systemic disease and therapeutic targets
Holistic Integrative Oncology (2024)
-
LCN2 secreted by tissue-infiltrating neutrophils induces the ferroptosis and wasting of adipose and muscle tissues in lung cancer cachexia
Journal of Hematology & Oncology (2023)