Abstract
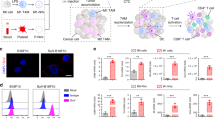
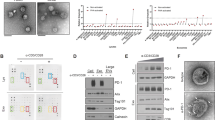
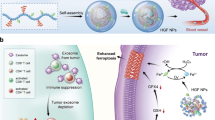
Impaired type I interferons (IFNs) may cause immune deficiency in tumours. Current supplementary IFN therapy partially restores anticancer immunity but simultaneously induces immune evasion by upregulating multiple immune checkpoints. Here we create a T lymphocyte membrane-decorated epigenetic nanoinducer that is engineered with programmed cell death protein 1 (PD1), which we call OPEN, for the delivery of the IFN inducer ORY-1001. OPEN increases IFNs and blocks IFN-induced immune checkpoint upregulation. OPEN also targets tumours that express programmed cell death ligand 1 (PDL1) through PDL1/PD1 recognition and subsequently triggers the internalization of OPEN and immune checkpoint proteins. OPEN, which is loaded with ORY-1001, upregulates intratumoural IFNs and downstream major histocompatibility complex I and PDL1. The replenished PDL1 enables further ligation of OPEN, which in turn blocks PDL1. These sequential processes result in an eight- and 29-fold increase of the intratumoural densities of total and active cytotoxic T lymphocytes, respectively, and a strong inhibition of xenograft tumour growth. This T lymphocyte membrane-decorated epigenetic nanoinducer presents a generalizable platform to boost antitumour immunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data are provided with this paper. Gene expression and survival data of TNBC patients in Fig. 2a were acquired from the cBioPortal database (www.cbioportal.org). The raw data used in Fig. 2g and Supplementary Fig. 2 are available at https://www.biosino.org/node with the dataset identifier number OEX010736. Any additional data that support the findings of this study are available from the corresponding authors upon reasonable request.
Code availability
All custom R scripts associated with this manuscript are deposited in the publicly available repository (https://gitee.com/ginwang/simm.git).
References
Dunn, G. P., Koebel, C. M. & Schreiber, R. D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6, 836–848 (2006).
Minn, A. J. & Wherry, E. J. Combination cancer therapies with immune checkpoint blockade: convergence on interferon signaling. Cell 165, 272–275 (2016).
Parker, B. S., Rautela, J. & Hertzog, P. J. Antitumour actions of interferons: implications for cancer therapy. Nat. Rev. Cancer 16, 131–144 (2016).
Zitvogel, L., Galluzzi, L., Kepp, O., Smyth, M. J. & Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 15, 405–414 (2015).
Sceneay, J. et al. Interferon signaling is diminished with age and is associated with immune checkpoint blockade efficacy in triple-negative breast cancer. Cancer Discov. 9, 1208–1227 (2019).
Critchley-Thorne, R. J. et al. Impaired interferon signaling is a common immune defect in human cancer. Proc. Natl Acad. Sci. USA 106, 9010–9015 (2009).
Sisirak, V. et al. Impaired IFN-alpha production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 72, 5188–5197 (2012).
Domschke, C. et al. Intratumoral cytokines and tumor cell biology determine spontaneous breast cancer-specific immune responses and their correlation to prognosis. Cancer Res. 69, 8420–8428 (2009).
Bidwell, B. N. et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 18, 1224–1231 (2012).
Salvagno, C. et al. Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response. Nat. Cell Biol. 21, 511–521 (2019).
Sistigu, A. et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 20, 1301–1309 (2014).
Benci, J. L. et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 167, 1540–1554 (2016).
Demaria, O. et al. Harnessing innate immunity in cancer therapy. Nature 574, 45–56 (2019).
Cauwels, A. et al. Delivering type I interferon to dendritic cells empowers tumor eradication and immune combination treatments. Cancer Res. 78, 463–474 (2018).
De Palma, M. et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell 14, 299–311 (2008).
Escobar, G. et al. Genetic engineering of hematopoiesis for targeted IFN-α delivery inhibits breast cancer progression. Sci. Transl. Med. 6, 217ra213 (2014).
Topper, M. J., Vaz, M., Marrone, K. A., Brahmer, J. R. & Baylin, S. B. The emerging role of epigenetic therapeutics in immuno-oncology. Nat. Rev. Clin. Oncol. 17, 75–90 (2020).
Sheng, W. Q. et al. LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell 174, 549–563 (2018).
Maes, T. et al. ORY-1001, a potent and selective covalent KDM1A inhibitor, for the treatment of acute leukemia. Cancer Cell 33, 495–511 (2018).
Poggio, M. et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 177, 414–427 (2019).
Hu, C. M. J. et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA 108, 10980–10985 (2011).
Hu, C. M. J., Fang, R. H., Luk, B. T. & Zhang, L. F. Nanoparticle-detained toxins for safe and effective vaccination. Nat. Nanotechnol. 8, 933–938 (2013).
Hu, C. M. J. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121 (2015).
Wang, C. et al. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat. Biomed. Eng. 1, 0011 (2017).
Hu, Q. Y. et al. Anticancer platelet-mimicking nanovehicles. Adv. Mater. 27, 7043–7050 (2015).
Ciriello, G. et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163, 506–519 (2015).
Rueda, O. M. et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature 567, 399–404 (2019).
Tokuyama, M. et al. ERVmap analysis reveals genome-wide transcription of human endogenous retroviruses. Proc. Natl Acad. Sci. USA 115, 12565–12572 (2018).
Kinter, A. L. et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J. Immunol. 181, 6738–6746 (2008).
Parry, R. V. et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 25, 9543–9553 (2005).
Jiang, W., Kim, B. Y. S., Rutka, J. T. & Chan, W. C. W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 3, 145–150 (2008).
Gillis, S. & Smith, K. A. Long term culture of tumour-specific cytotoxic T cells. Nature 268, 154–156 (1977).
Li, C. W. et al. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell 33, 187–201 (2018).
Kalbasi, A. et al. Uncoupling interferon signaling and antigen presentation to overcome immunotherapy resistance due to JAK1 loss in melanoma. Sci. Transl. Med. 12, eabb0152 (2020).
Seliger, B., Wollscheid, U., Momburg, F., Blankenstein, T. & Huber, C. Characterization of the major histocompatibility complex class I deficiencies in B16 melanoma cells. Cancer Res. 61, 1095–1099 (2001).
Tang, L. et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 36, 707–716 (2018).
Schmidt-Arras, D. & Rose-John, S. IL-6 pathway in the liver: from physiopathology to therapy. J. Hepatol. 64, 1403–1415 (2016).
Garrido-Castro, A. C., Lin, N. U. & Polyak, K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 9, 176–198 (2019).
Bareche, Y. et al. Unraveling triple-negative breast cancer tumor microenvironment heterogeneity: towards an optimized treatment approach. J. Natl Cancer Inst. 112, 708–719 (2020).
Savas, P. et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat. Rev. Clin. Oncol. 13, 228–241 (2016).
Gruosso, T. et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J. Clin. Invest. 129, 1785–1800 (2019).
Zhang, X. et al. PD-1 blockade cellular vesicles for cancer immunotherapy. Adv. Mater. 30, 1707112 (2018).
Zhang, X. et al. Engineering PD-1-presenting platelets for cancer immunotherapy. Nano Lett. 18, 5716–5725 (2018).
Ferguson, S. S. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 53, 1–24 (2001).
Sagiv-Barfi, I. et al. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc. Natl Acad. Sci. USA 112, E966–E972 (2015).
de Graauw, M. et al. Annexin A1 regulates TGF-beta signaling and promotes metastasis formation of basal-like breast cancer cells. Proc. Natl Acad. Sci. USA 107, 6340–6345 (2010).
Spranger, S., Bao, R. Y. & Gajewski, T. F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015).
Bald, T. et al. Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Discov. 4, 674–687 (2014).
Rafiq, S., Hackett, C. S. & Brentjens, R. J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 17, 147–167 (2020).
Acknowledgements
Y.L. thanks the National Natural Science Foundation of China (81690265) for financial support. P.Z. thanks the National Natural Science Foundation of China (31870995 and 81671808), the Youth Innovation Promotion Association of CAS (2017335) and the SA-SIBS Scholarship Program for financial support. Y.L. thanks the Shandong Provincial Natural Science Foundation (ZR2019ZD25) for financial support. We are grateful to the National Centre for Protein Science Shanghai (electron microscopy system) for instrument support and technical assistance during data collection. We also thank L. Deng at the Shanghai Institute of Immunology for the kind gift of OT I mice and we thank J. Wang at Shanghai Institute of Materia Medica for the help with linker synthesis.
Author information
Authors and Affiliations
Contributions
P.Z. and Y.Z. conceived and designed the project; Y.Z. synthesized and characterized the nanovesicles; Y.Z., J.W., T.L., Y.K., R.R., Y.C., W.R., F.X., C.Z., Y.W. and Y.Y. performed the cell and animal experiments; J.W., H.H.Z. and P.Z. performed bioinformatic analysis; P.Z., Y.Z. and Y.L. interpreted the data and wrote the manuscript with input from all of the authors. P.Z. and Y.L. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Riccardo Dolcetti, Liangfang Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–40 and ref. 1.
Supplementary Data1
Statistical source data for the supplementary figures.
Source data
Source Data Fig. 2
Statistical source data and unprocessed western blots.
Source Data Fig. 3
Statistical source data and unprocessed gel and western blot.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
About this article
Cite this article
Zhai, Y., Wang, J., Lang, T. et al. T lymphocyte membrane-decorated epigenetic nanoinducer of interferons for cancer immunotherapy. Nat. Nanotechnol. 16, 1271–1280 (2021). https://doi.org/10.1038/s41565-021-00972-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-00972-7
This article is cited by
-
LSD1: an emerging face in altering the tumor microenvironment and enhancing immune checkpoint therapy
Journal of Biomedical Science (2023)
-
Cholesterol removal improves performance of a model biomimetic system to co-deliver a photothermal agent and a STING agonist for cancer immunotherapy
Nature Communications (2023)
-
Interventional hydrogel microsphere vaccine as an immune amplifier for activated antitumour immunity after ablation therapy
Nature Communications (2023)
-
Targeting drugs to tumours using cell membrane-coated nanoparticles
Nature Reviews Clinical Oncology (2023)
-
Microbial synthesis of Prussian blue for potentiating checkpoint blockade immunotherapy
Nature Communications (2023)