Key Points
-
Small-cell carcinoma (SCC) of the prostate is a rare subtype of prostate cancer characterized by an aggressive clinical course
-
Approximately 40–50% of men with prostatic SCCs have a history of conventional prostatic adenocarcinoma
-
SCC of the prostate is usually not responsive to androgen deprivation and disease progression is not associated with rises in serum PSA levels
-
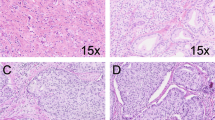
A biopsy of accessible lesions should be considered when SCC is suspected
-
Chemotherapy represents the backbone of management for men with advanced prostatic SCC
-
SCC is radiosensitive and radiotherapy might offer local palliation of symptoms such as bladder outlet obstruction and pain
Abstract
Pure small-cell carcinoma (SCC) of the prostate is a rare entity and one of the most aggressive malignancies of the prostate. Histologically, prostatic SCCs of the prostate are part of a spectrum of anaplastic tumours of the prostate and are similar to SCCs of the lungs. In most cases, SCC of the prostate is associated with conventional prostatic adenocarcinoma. Both components of these mixed tumours frequently share molecular alterations such as ERG gene rearrangements or AURKA and MYCN amplifications, suggesting a common clonal origin. The clinical behaviour of small-cell prostate carcinomas is characterized by extensive local disease, visceral disease, and low PSA levels despite large metastatic burden. Commonly, the emergence of the SCC occurs in patients with high-grade adenocarcinoma who are often treated with androgen deprivation treatment (ADT). However, SCCs do not usually benefit from ADT. A biopsy of accessible lesions is strongly recommended to identify those with SCC pathological features, as management is undoubtedly affected by this finding. Chemotherapy is the standard approach for treating patients with either localized or advanced prostatic SCC. Despite the emergence of more-aggressive treatment modalities, the prognosis of men with prostatic SCC remains dismal.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
di Sant'Agnese, P. A. & Cockett, A. T. Neuroendocrine differentiation in prostatic malignancy. Cancer 78, 357–361 (1996).
di Sant'Agnese, P. A. Neuroendocrine differentiation in prostatic carcinoma: an update on recent developments. Ann. Oncol. 12 (Suppl. 2), S135–S140 (2001).
Casella, R. et al. Focal neuroendocrine differentiation lacks prognostic significance in prostate core needle biopsies. J. Urol. 160, 406–410 (1998).
Aprikian, A. G., Cordon-Cardo, C., Fair, W. R. & Reuter, V. E. Characterization of neuroendocrine differentiation in human benign prostate and prostatic adenocarcinoma. Cancer 71, 3952–3965 (1993).
Cohen, R. J., Glezerson, G. & Haffejee, Z. Neuro-endocrine cells—a new prognostic parameter in prostate cancer. Br. J. Urol. 68, 258–262 (1991).
Shariff, A. H. & Ather, M. H. Neuroendocrine differentiation in prostate cancer. Urology 68, 2–8 (2006).
Abrahamsson, P. A., Cockett, A. T. & di Sant'Agnese, P. A. Prognostic significance of neuroendocrine differentiation in clinically localized prostatic carcinoma. Prostate Suppl. 8, 37–42 (1998).
Allen, F. J., Van Velden, D. J. & Heyns, C. F. Are neuroendocrine cells of practical value as an independent prognostic parameter in prostate cancer? Br. J. Urol. 75, 751–754 (1995).
Ahlgren, G. et al. Regressive changes and neuroendocrine differentiation in prostate cancer after neoadjuvant hormonal treatment. Prostate 42, 274–279 (2000).
Tan, M. O. et al. Prostate cancer and neuroendocrine differentiation. Int. Urol. Nephrol. 31, 75–82 (1999).
Cohen, M. K. et al. Neuroendocrine differentiation in prostatic adenocarcinoma and its relationship to tumour progression. Cancer 74, 1899–1903 (1994).
Ishida, E., Nakamura, M., Shimada, K., Tasaki, M. & Konishi, N. Immunohistochemical analysis of neuroendocrine differentiation in prostate cancer. Pathobiology 76, 30–38 (2009).
Jeetle, S. S. et al. Neuroendocrine differentiation does not have independent prognostic value in conservatively treated prostate cancer. Virchows Arch. 461, 103–107 (2012).
Aprikian, A. G. et al. Neuroendocrine differentiation in metastatic prostatic adenocarcinoma. J. Urol. 151, 914–919 (1994).
Wenk, R. E., Bhagavan, B. S., Levy, R., Miller, D. & Weisburger, W. Ectopic ACTH, prostatic oat cell carcinoma, and marked hypernatraemia. Cancer 40, 773–778 (1977).
Miyoshi, Y. et al. Neuroendocrine differentiated small cell carcinoma presenting as recurrent prostate cancer after androgen deprivation therapy. BJU Int. 88, 982–983 (2001).
Tanaka, M. et al. Progression of prostate cancer to neuroendocrine cell tumour. Int. J. Urol. 8, 431–437 (2001).
Turbat-Herrera, E. A. et al. Neuroendocrine differentiation in prostatic carcinomas. A retrospective autopsy study. Arch. Pathol. Lab. Med. 112, 1100–1105 (1988).
Shah, R. B. et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy programme. Cancer Res. 64, 9209–9216 (2004).
Tetu, B. et al. Small cell carcinoma of the prostate. Part I. A clinicopathologic study of 20 cases. Cancer 59, 1803–1809 (1987).
Yao, J. L. et al. Small cell carcinoma of the prostate: an immunohistochemical study. Am. J. Surg. Pathol. 30, 705–712 (2006).
Ro, J. Y., Tetu, B., Ayala, A. G. & Ordonez, N. G. Small cell carcinoma of the prostate. II. Immunohistochemical and electron microscopic studies of 18 cases. Cancer 59, 977–982 (1987).
Wang, W. & Epstein, J. I. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am. J. Surg. Pathol. 32, 65–71 (2008).
Agoff, S. N. et al. Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumours. Mod. Pathol. 13, 238–242 (2000).
Ordonez, N. G. Value of thyroid transcription factor-1, E-cadherin, BG8, WT1, and CD44S immunostaining in distinguishing epithelial pleural mesothelioma from pulmonary and nonpulmonary adenocarcinoma. Am. J. Surg. Pathol. 24, 598–606 (2000).
Lotan, T. L. et al. ERG gene rearrangements are common in prostatic small cell carcinomas. Mod. Pathol. 24, 820–828 (2011).
Han, B. et al. Characterization of ETS gene aberrations in select histologic variants of prostate carcinoma. Mod. Pathol. 22, 1176–1185 (2009).
Guo, C. C. et al. TMPRSS2-ERG gene fusion in small cell carcinoma of the prostate. Hum. Pathol. 42, 11–17 (2011).
Williamson, S. R. et al. ERG-TMPRSS2 rearrangement is shared by concurrent prostatic adenocarcinoma and prostatic small cell carcinoma and absent in small cell carcinoma of the urinary bladder: evidence supporting monoclonal origin. Mod. Pathol. 24, 1120–1127 (2011).
Scheble, V. J. et al. ERG rearrangement in small cell prostatic and lung cancer. Histopathology 56, 937–943 (2011).
Schelling, L. A. et al. Frequent TMPRSS2-ERG rearrangement in prostatic small cell carcinoma detected by fluorescence in situ hybridization: the superiority of fluorescence in situ hybridization over ERG immunohistochemistry. Hum. Pathol. 44, 2227–2233 (2013).
Evans, A. J., Humphrey, P. A., Belani, J., van der Kwast, T. H. & Srigley, J. R. Large cell neuroendocrine carcinoma of prostate: a clinicopathologic summary of 7 cases of a rare manifestation of advanced prostate cancer. Am. J. Surg. Pathol. 30, 684–693 (2006).
Pearse, A. G. The diffuse endocrine system and the implications of the APUD concept. Int. Surg. 64, 5–7 (1979).
Oesterling, J. E., Hauzeur, C. G. & Farrow, G. M. Small cell anaplastic carcinoma of the prostate: a clinical, pathological and immunohistological study of 27 patients. J. Urol. 147, 804–807 (1992).
Ismail, A. H., Landry, F., Aprikian, A. G. & Chevalier, S. Androgen ablation promotes neuroendocrine cell differentiation in dog and human prostate. Prostate 51, 117–125 (2002).
Wright, M. E., Tsai, M. J. & Aebersold, R. Androgen receptor represses the neuroendocrine transdifferentiation process in prostate cancer cells. Mol. Endocrinol. 17, 1726–1737 (2003).
Yuan, T. C. et al. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocr. Relat. Cancer 13, 151–167 (2006).
Mosquera, J. M. et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia 15, 1–10 (2013).
Spiess, P. E. et al. Treatment outcomes of small cell carcinoma of the prostate: a single-centre study. Cancer 110, 1729–1737 (2007).
Helpap, B., Kollermann, J. & Oehler, U. Neuroendocrine differentiation in prostatic carcinomas: histogenesis, biology, clinical relevance, and future therapeutical perspectives. Urol. Int. 62, 133–138 (1999).
Bonkhoff, H. & Remberger, K. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. Prostate 28, 98–106 (1996).
Stratton, M., Evans, D. J. & Lampert, I. A. Prostatic adenocarcinoma evolving into carcinoid: selective effect of hormonal treatment? J. Clin. Pathol. 39, 750–756 (1986).
Burchardt, T. et al. Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. J. Urol. 162, 1800–1805 (1999).
Scheble, V. J. et al. ERG rearrangement is specific to prostate cancer and does not occur in any other common tumour. Mod. Pathol. 23, 1061–1067 (2010).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Lapuk, A. V. et al. From sequence to molecular pathology, and a mechanism driving the neuroendocrine phenotype in prostate cancer. J. Pathol. 227, 286–297 (2012).
Hansel, D. E. et al. Shared TP53 gene mutation in morphologically and phenotypically distinct concurrent primary small cell neuroendocrine carcinoma and adenocarcinoma of the prostate. Prostate 69, 603–609 (2009).
Beltran, H. et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 1, 487–495 (2011).
Clegg, N. et al. Molecular characterization of prostatic small-cell neuroendocrine carcinoma. Prostate 55, 55–64 (2003).
Tzelepi, V. et al. Modeling a lethal prostate cancer variant with small-cell carcinoma features. Clin. Cancer Res. 18, 666–677 (2012).
Palmgren, J. S., Karavadia, S. S. & Wakefield, M. R. Unusual and underappreciated: small cell carcinoma of the prostate. Semin. Oncol. 34, 22–29 (2007).
Abrahamsson, P. A. Neuroendocrine cells in tumour growth of the prostate. Endocr. Relat. Cancer 6, 503–519 (1999).
Alshaikh, O. M., Al-Mahfouz, A. A., Al-Hindi, H., Mahfouz, A. B. & Alzahrani, A. S. Unusual cause of ectopic secretion of adrenocorticotropic hormone: Cushing syndrome attributable to small cell prostate cancer. Endocr. Pract. 16, 249–254 (2010).
Aparicio, A. M. et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin. Cancer Res. 19, 3621–3630 (2013).
Flechon, A. et al. Phase II study of carboplatin and etoposide in patients with anaplastic progressive metastatic castration-resistant prostate cancer (mCRPC) with or without neuroendocrine differentiation: results of the French Genito-Urinary Tumour Group (GETUG) P01 trial. Ann. Oncol. 22, 2476–2481 (2011).
Loriot, Y. et al. Combining carboplatin and etoposide in docetaxel-pretreated patients with castration-resistant prostate cancer: a prospective study evaluating also neuroendocrine features. Ann. Oncol. 20, 703–708 (2009).
Culine, S. et al. Docetaxel and cisplatin in patients with metastatic androgen independent prostate cancer and circulating neuroendocrine markers. J. Urol. 178, 844–848 (2007).
McCutcheon, I. E., Eng, D. Y. & Logothetis, C. J. Brain metastasis from prostate carcinoma: antemortem recognition and outcome after treatment. Cancer 86, 2301–2311 (1999).
Cussenot, O., Villette, J. M., Cochand-Priollet, B. & Berthon, P. Evaluation and clinical value of neuroendocrine differentiation in human prostatic tumours. Prostate Suppl. 8, 43–51 (1998).
Heinrich, E. et al. Neuroendocrine tumour cells in prostate cancer: evaluation of the neurosecretory products 5-hydroxytryptamine, bombesin, and gastrin—impact on angiogenesis and clinical follow-up. Prostate 71, 1752–1758 (2011).
Ferrero-Pous, M., Hersant, A. M., Pecking, A., Bresard-Leroy, M. & Pichon, M. F. Serum chromogranin-A in advanced prostate cancer. BJU Int. 88, 790–796 (2001).
Amato, R. J. et al. Chemotherapy for small cell carcinoma of prostatic origin. J. Urol. 147, 935–937 (1992).
Hindson, D. A., Knight, L. L. & Ocker, J. M. Small-cell carcinoma of prostate. Transient complete remission with chemotherapy. Urology 26, 182–184 (1985).
Steineck, G. et al. Cytotoxic treatment of aggressive prostate tumours with or without neuroendocrine elements. Acta Oncol. 41, 668–674 (2002).
Papandreou, C. N. et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J. Clin. Oncol. 20, 3072–3080 (2002).
Deorah, S., Rao, M. B., Raman, R., Gaitonde, K. & Donovan, J. F. Survival of patients with small cell carcinoma of the prostate during 1973–2003: a population-based study. BJU Int. 109, 824–830 (2012).
Bolton, D. M., Chiu, S. T., Clarke, S. & Angus, D. Primary small cell carcinoma of the prostate: unusual modes of presentation. Aust. N. Z. J. Surg. 64, 91–94 (1994).
Kim, K. H., Kim, Y. B., Lee, J. K., Kim, Y. J. & Jung, T. Y. Pathologic results of radical prostatectomies in patients with simultaneous atypical small acinar proliferation and prostate cancer. Korean J. Urol. 51, 398–402 (2010).
Sule-Suso, J. & Brunt, A. M. Small cell carcinoma of the prostate. Br. J. Radiol. 65, 726–728 (1992).
NCC guidelines Index: Version 2.2013. Prostate Cancer [online], (2013).
Auperin, A. et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N. Engl. J. Med. 341, 476–484 (1999).
Rubenstein, J. H. et al. Small cell anaplastic carcinoma of the prostate: seven new cases, review of the literature, and discussion of a therapeutic strategy. Am. J. Clin. Oncol. 20, 376–380 (1997).
Moore, S. R., Reinberg, Y. & Zhang, G. Small cell carcinoma of prostate: effectiveness of hormonal versus chemotherapy. Urology 39, 411–416 (1992).
NCC guidelines Index: Version 2.2013. Adult cancer pain [online], (2013).
Janssen, A. & Moedema, R. H. Mitosis as an anti-cancer target. Oncogene 30, 2799–2809 (2011).
Stein, M. E. et al. Small cell (neuroendocrine) carcinoma of the prostate: aetiology, diagnosis, prognosis, and therapeutic implications—a retrospective study of 30 patients from the rare cancer network. Am. J. Med. Sci. 336, 478–488 (2008).
Author information
Authors and Affiliations
Contributions
R.N., M.S., and O.N.K. researched data and were involved in writing the article. J.I.E. and M.A.E. contributed to discussions of content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nadal, R., Schweizer, M., Kryvenko, O. et al. Small cell carcinoma of the prostate. Nat Rev Urol 11, 213–219 (2014). https://doi.org/10.1038/nrurol.2014.21
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2014.21
This article is cited by
-
Second generation anti-androgens and androgen deprivation therapy with radiation therapy in the definitive management of high-risk prostate cancer
Prostate Cancer and Prostatic Diseases (2023)
-
ACAA2 is a novel molecular indicator for cancers with neuroendocrine phenotype
British Journal of Cancer (2023)
-
Neuroendocrine neoplasms of the lung and gastrointestinal system: convergent biology and a path to better therapies
Nature Reviews Clinical Oncology (2023)
-
Oral formulation of bendamustine hydrochloride for patients with advanced solid tumors; a phase 1 study
Investigational New Drugs (2023)
-
Small-Cell Carcinoma of the Prostate – Challenges of Diagnosis and Treatment: A Next of Kin and Physician Perspective Piece
Oncology and Therapy (2023)