Key Points
-
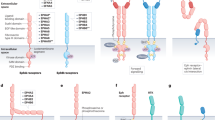
Transforming growth factor-β (TGFβ) signalling is mediated by TGFβ ligands, type 1 and type 2 receptors, and Smad proteins. TGFβ also regulates non-Smad pathways.
-
TGFβ stimulation inhibits cancer cell proliferation in some cellular contexts and promotes it in others. Numerous factors are involved in TGFβ-regulated cell proliferation and keep its signalling pathways balanced.
-
In addition to perturbation of TGFβ signalling, disruption or mutation of regulators of TGFβ signalling can lead to a loss of balanced TGFβ signalling, resulting in the generation and progression of tumours.
-
TGFβ signalling in cancer cells has dual roles in the regulation of cell death and proliferation.
-
TGFβ signalling has crucial roles in the maintenance of self-renewal and tumorigenic activity of glioma-initiating cells and leukaemia-initiating cells, whereas the function of TGFβ signalling in breast cancer-initiating cells is controversial.
-
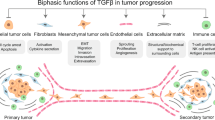
TGFβ signalling is involved in several cell responses during cancer cell metastasis, and cell type-dependent and context-dependent factors contribute to the regulation of tumour metastasis.
-
The TGFβ pathway has been targeted for cancer therapy using multiple strategies. Some of them are currently in clinical trials.
Abstract
The distortion of growth factor signalling is the most important prerequisite in tumour progression. Transforming growth factor-β (TGFβ) signalling regulates tumour progression by a tumour cell-autonomous mechanism or through tumour–stroma interaction, and has either a tumour-suppressing or tumour-promoting function depending on cellular context. Such inherent complexity of TGFβ signalling results in arduous, but promising, assignments for developing therapeutic strategies against malignant tumours. As numerous cellular context-dependent factors tightly maintain the balance of TGFβ signalling and contribute to the regulation of TGFβ-induced cell responses, in this Review we discuss how they maintain the balance of TGFβ signalling and how their collapse leads to tumour progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Blobe, G. C., Schiemann, W. P. & Lodish, H. F. Role of transforming growth factor β in human disease. N. Engl. J. Med. 342, 1350–1358 (2000).
Levy, L. & Hill, C. S. Alterations in components of the TGF-β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 17, 41–58 (2006).
Markowitz, S. et al. Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science 268, 1336–1338 (1995).
Bornstein, S. et al. Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J. Clin. Invest. 119, 3408–3419 (2009).
Tsushima, H. et al. High levels of transforming growth factor β1 in patients with colorectal cancer: association with disease progression. Gastroenterology 110, 375–382 (1996).
Wikström, P., Stattin, P., Franck-Lissbrant, I., Damber, J. E. & Bergh, A. Transforming growth factor β1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate 37, 19–29 (1998).
Walker, R. A. & Dearing, S. J. Transforming growth factor β1 in ductal carcinoma in situ and invasive carcinomas of the breast. Eur. J. Cancer 28, 641–644 (1992).
Friedman, E. et al. High levels of transforming growth factor β1 correlate with disease progression in human colon cancer. Cancer Epidemiol. Biomarkers Prev. 4, 549–554 (1995).
Dalal, B. I., Keown, P. A. & Greenberg, A. H. Immunocytochemical localization of secreted transforming growth factor-β1 to the advancing edges of primary tumors and to lymph node metastases of human mammary carcinoma. Am. J. Pathol. 143, 381–389 (1993).
Picon, A., Gold, L. I., Wang, J., Cohen, A. & Friedman, E. A subset of metastatic human colon cancers expresses elevated levels of transforming growth factor β1. Cancer Epidemiol. Biomarkers Prev. 7, 497–504 (1998).
Bierie, B. & Moses, H. L. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nature Rev. Cancer 6, 506–520 (2006). This Review covers the roles of TGFβ signalling in the tumour microenvironment and discusses the paradoxical effects of this cytokine in tumour progression: tumour promotion compared with tumour suppression.
Li, M. O., Wan, Y. Y., Sanjabi, S., Robertson, A. K. & Flavell, R. A. Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol. 24, 99–146 (2006).
Heldin, C. H., Miyazono, K. & ten Dijke, P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390, 465–471 (1997).
Shi, Y. & Massague, J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113, 685–700 (2003).
Feng, X. H. & Derynck, R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 21, 659–693 (2005).
Annes, J. P., Munger, J. S. & Rifkin, D. B. Making sense of latent TGFβ activation. J. Cell Sci. 116, 217–224 (2003).
Rifkin, D. B. Latent transforming growth factor-β (TGF-β) binding proteins: orchestrators of TGF-β availability. J. Biol. Chem. 280, 7409–7412 (2005).
Goumans, M. J. et al. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFβ/ALK5 signaling. Mol. Cell 12, 817–828 (2003).
Daly, A. C., Randall, R. A. & Hill, C. S. Transforming growth factor β-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol. Cell. Biol. 28, 6889–6902 (2008).
Derynck, R. & Zhang, Y. E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 (2003).
Miyazawa, K., Shinozaki, M., Hara, T., Furuya, T. & Miyazono, K. Two major Smad pathways in TGF-β superfamily signalling. Genes Cells 7, 1191–1204 (2002).
Koinuma, D. et al. Chromatin immunoprecipitation on microarray analysis of Smad2/3 binding sites reveals roles of ETS1 and TFAP2A in transforming growth factor β signaling. Mol. Cell. Biol. 29, 172–186 (2009).
Koinuma, D. et al. Promoter-wide analysis of Smad4 binding sites in human epithelial cells. Cancer Sci. 100, 2133–2142 (2009).
Lin, X. Chen, Y. G. & Feng, X. H. in The TGF-β Family (eds Derynck, R. & Miyazono, K.) 287–332 (Cold Spring Harbor Laboratory Press, New York, 2008).
Ikushima, H. et al. An Id-like molecule, HHM, is a synexpression group-restricted regulator of TGF-β signalling. EMBO J. 27, 2955–2965 (2008). This paper suggests a new mechanism for regulating some of the TGFβ-induced cellular responses. HHM targets some of the Smad-binding transcriptional cofactors and regulates TGFβ signalling in a synexpression group-selective manner.
Massague, J. TGFβ in cancer. Cell 134, 215–230 (2008).
Ikushima, H. & Miyazono, K. Cellular context-dependent “colors” of transforming growth factor-β signaling. Cancer Sci. 101, 306–312 (2010).
Janknecht, R., Wells, N. J. & Hunter, T. TGF-β-stimulated cooperation of Smad proteins with the coactivators CBP/p300. Genes Dev. 12, 2114–2119 (1998).
Feng, X. H., Zhang, Y., Wu, R. Y. & Derynck, R. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for Smad3 in TGF-β-induced transcriptional activation. Genes Dev. 12, 2153–2163 (1998).
Chen, C. R., Kang, Y., Siegel, P. M. & Massague, J. E2F4/5 and p107 as Smad cofactors linking the TGFβ receptor to c-myc repression. Cell 110, 19–32 (2002).
Moustakas, A. & Heldin, C. H. Non-Smad TGF-β signals. J. Cell Sci. 118, 3573–3584 (2005).
Zhang, Y. E. Non-Smad pathways in TGF-β signaling. Cell Res. 19, 128–139 (2009).
Datto, M. B. et al. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc. Natl Acad. Sci. USA 92, 5545–5549 (1995).
Hannon, G. J. & Beach, D. p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature 371, 257–261 (1994).
Yagi, K. et al. c-myc is a downstream target of the Smad pathway. J. Biol. Chem. 277, 854–861 (2002).
Azar, R., Alard, A., Susini, C., Bousquet, C. & Pyronnet, S. 4E-BP1 is a target of Smad4 essential for TGFβ-mediated inhibition of cell proliferation. EMBO J. 28, 3514–3522 (2009).
Deheuninck, J. & Luo, K. Ski and SnoN, potent negative regulators of TGF-β signaling. Cell Res. 19, 47–57 (2009). This review discusses recent findings on the biological functions of SKI and SKIL and their mechanisms of action. It also addresses how expression levels of these factors are regulated.
Suzuki, H. et al. c-Ski inhibits the TGF-β signaling pathway through stabilization of inactive Smad complexes on Smad-binding elements. Oncogene 23, 5068–5076 (2004).
Pan, D., Zhu, Q. & Luo, K. SnoN functions as a tumour suppressor by inducing premature senescence. EMBO J. 28, 3500–3513 (2009).
Zhu, Q. et al., Dual role of SnoN in mammalian tumorigenesis. Mol. Cell. Biol. 27, 324–339 (2007).
Heider, T. R., Lyman, S., Schoonhoven, R. & Behrns, K. E. Ski promotes tumor growth through abrogation of transforming growth factor-β signaling in pancreatic cancer. Ann. Surg. 246, 61–68 (2007).
Morishita, K. et al. Activation of EVI1 gene expression in human acute myelogenous leukemias by translocations spanning 300–400 kilobases on chromosome band 3q26. Proc. Natl Acad. Sci. USA. 89, 3937–3941 (1992).
Kurokawa, M. et al. The oncoprotein Evi-1 represses TGF-β signalling by inhibiting Smad3. Nature 394, 92–96 (1998).
Mochizuki, N. et al. A novel gene, MEL1, mapped to 1p36.3 is highly homologous to the MDS1/EVI1 gene and is transcriptionally activated in t(1;3)(p36;q21)-positive leukemia cells. Blood 96, 3209–3214 (2000).
Takahata, M. et al. SKI and MEL1 cooperate to inhibit transforming growth factor-β signal in gastric cancer cells. J. Biol. Chem. 284, 3334–3344 (2009). This paper reports that MEL1, a novel regulator of SKI, stabilizes an inactive SMAD3–SKI complex on the promoter of TGFβ target genes and inhibits TGFβ signalling in gastric cancer cells.
Ohno, H. Pathogenetic role of BCL6 translocation in B-cell non-Hodgkin's lymphoma. Histol. Histopathol. 19, 637–650 (2004).
Pasqualucci, L. et al. Molecular pathogenesis of non-Hodgkin's lymphoma: the role of Bcl-6. Leuk. Lymphoma 44, S5–S12 (2003).
Wang, D. et al. BCL6 represses Smad signaling in transforming growth factor-β resistance. Cancer Res. 68, 783–789 (2008). This paper indicates that overexpression of BCL6 contributes to the resistance of B cell lymphoma to TGFβ-mediated growth inhibition.
Mori, N. et al. Human T-cell leukemia virus type I oncoprotein Tax represses Smad-dependent transforming growth factor β signaling through interaction with CREB-binding protein/p300. Blood 97, 2137–2144 (2001).
Lee, D. K., Kim, B. C., Brady, J. N., Jeang, K. T. & Kim, S. J. Human T-cell lymphotropic virus type 1 tax inhibits transforming growth factor-β signaling by blocking the association of Smad proteins with Smad-binding element. J. Biol. Chem. 277, 33766–33775 (2002).
Ito, Y. & Miyazono, K. RUNX transcription factors as key targets of TGF-β superfamily signaling. Curr. Opin. Genet. Dev. 13, 43–47 (2003).
Chi, X. Z. et al. RUNX3 suppresses gastric epithelial cell growth by inducing p21WAF1/Cip1 expression in cooperation with transforming growth factor β-activated SMAD. Mol. Cell. Biol. 25, 8097–8107 (2005).
Yano, T. et al. The RUNX3 tumor suppressor upregulates Bim in gastric epithelial cells undergoing transforming growth factor β-induced apoptosis. Mol. Cell. Biol. 26, 4474–4488 (2006).
Battegay, E. J., Raines, E. W., Seifert, R. A., Bowen-Pope, D. F. & Ross, R. TGF-β induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell 63, 515–524 (1990).
Bruna, A. et al. High TGFβ-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell 11, 147–160 (2007). This study demonstrates the pathological importance of epigenetic regulation of TGFβ signalling through methylation of Smad-binding elements in CpG islands of TGFβ target genes.
Matsuyama, S. et al. SB-431542 and Gleevec inhibit transforming growth factor-β-induced proliferation of human osteosarcoma cells. Cancer Res. 63, 7791–7798 (2003).
Lu, Q. R. et al. Oligodendrocyte lineage genes (OLIG) as molecular markers for human glial brain tumors. Proc. Natl Acad. Sci. USA 98, 10851–10856 (2001).
Jang, C. W. et al. TGF-β induces apoptosis through Smad-mediated expression of DAP-kinase. Nature Cell Biol. 4, 51–58 (2002).
Takekawa, M. et al. Smad-dependent GADD45β expression mediates delayed activation of p38 MAP kinase by TGF-β. EMBO J. 21, 6473–6482 (2002).
Ohgushi, M. et al. Transforming growth factor β-dependent sequential activation of Smad, Bim, and caspase-9 mediates physiological apoptosis in gastric epithelial cells. Mol. Cell. Biol. 25, 10017–10028 (2005).
Egle, A., Harris, A. W., Bouillet, P. & Cory, S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc. Natl Acad. Sci. USA 101, 6164–6169 (2004).
Ehata, S. et al., Transforming growth factor-β promotes survival of mammary carcinoma cells through induction of antiapoptotic transcription factor DEC1. Cancer Res. 67, 9694–9703 (2007).
Currie, M. J. et al. Expression of vascular endothelial growth factor D is associated with hypoxia inducible factor (HIF-1α) and the HIF-1α target gene DEC1, but not lymph node metastasis in primary human breast carcinomas. J. Clin. Pathol. 57, 829–834 (2004).
Chakrabarti, J. et al. The transcription factor DEC1 (stra13, SHARP2) is associated with the hypoxic response and high tumour grade in human breast cancers. Br. J. Cancer 91, 954–958 (2004).
Kiyono, K. et al. Autophagy is activated by TGF-β and potentiates TGF-β-mediated growth inhibition in human hepatocellular carcinoma cells. Cancer Res. 69, 8844–8852 (2009).
Eisenberg-Lerner, A. & Kimchi, A. The paradox of autophagy and its implication in cancer etiology and therapy. Apoptosis 14, 376–391 (2009).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001).
Iwasaki, H. & Suda, T. Cancer stem cells and their niche. Cancer Sci. 100, 1166–1172 (2009).
Yamazaki, S. et al. TGF-β as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood 113, 1250–1256 (2009).
Tang, B. et al. Transforming growth factor-β can suppress tumorigenesis through effects on the putative cancer stem or early progenitor cell and committed progeny in a breast cancer xenograft model. Cancer Res. 67, 8643–8652 (2007).
Mani, S. A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008). This paper is the first to report a link between EMT and stem cell characteristics.
Watabe, T. & Miyazono, K. Roles of TGF-β family signaling in stem cell renewal and differentiation. Cell Res. 19, 103–115 (2009).
Golestaneh, N. & Mishra, B. TGF-β, neuronal stem cells and glioblastoma. Oncogene 24, 5722–5730 (2005).
Peñuelas, S. et al. TGF-β increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell 15, 315–327 (2009).
Ikushima, H. et al. Autocrine TGF-β signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell 5, 504–514 (2009). References 74 and 75 provide evidence that TGFβ signalling has crucial roles in the maintenance of self-renewal and tumorigenicity of glioma-initiating cells.
Kamachi, Y., Uchikawa, M. & Kondoh, H. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 16, 182–187 (2000).
Graham, V., Khudyakov, J., Ellis, P. & Pevny, L. SOX2 functions to maintain neural progenitor identity. Neuron 39, 749–765 (2003).
Ferri, A. L. et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 131, 3805–3819 (2004).
Bao, S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006).
Liu, G. et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer 5, 67 (2006).
Hau, P. et al. Inhibition of TGF-β2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides 17, 201–212 (2007).
Lim, D. A. et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28, 713–726 (2000).
Piccirillo, S. G. et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444, 761–765 (2006).
Lee, J. et al. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell 13, 69–80 (2008).
Naka, K. et al. TGF-β-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature 463, 676–680 (2010). This study shows that TGFβ signalling has a crucial role in the maintenance of leukaemia-initiating cells. It suggests that inhibition of the TGFβ pathway might represent a new therapeutic approach for patients with CML.
Hasegawa, Y. et al. Transforming growth factor-β1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer 91, 964–971 (2001).
Tuxhorn, J. A., McAlhany, S. J., Yang, F., Dang, T. D. & Rowley, D. R. Inhibition of transforming growth factor-β activity decreases angiogenesis in a human prostate cancer-reactive stroma xenograft model. Cancer Res. 62, 6021–6025 (2002).
Stearns, M. E., Garcia, F. U., Fudge, K., Rhim, J. & Wang, M. Role of interleukin 10 and transforming growth factor β1 in the angiogenesis and metastasis of human prostate primary tumor lines from orthotopic implants in severe combined immunodeficiency mice. Clin. Cancer Res. 5, 711–720 (1999).
Ueki, N. et al. Excessive production of transforming growth-factor β 1 can play an important role in the development of tumorigenesis by its action for angiogenesis: validity of neutralizing antibodies to block tumor growth. Biochim. Biophys. Acta. 1137, 189–196 (1992).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Sánchez-Elsner, T. et al. Synergistic cooperation between hypoxia and transforming growth factor-β pathways on human vascular endothelial growth factor gene expression. J. Biol. Chem. 276, 38527–38535 (2001).
Derynck, R., Akhurst, R. J. & Balmain, A. TGF-β signaling in tumor suppression and cancer progression. Nature Genet. 29, 117–129 (2001).
Schwarte-Waldhoff, I. et al. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc. Natl Acad. Sci. USA 97, 9624–9629 (2000).
Komuro, A. et al. Diffuse-type gastric carcinoma: progression, angiogenesis, and transforming growth factor β signaling. J. Natl. Cancer Inst. 101, 592–604 (2009).
Kiyono, K. et al. c-Ski overexpression promotes tumor growth and angiogenesis through inhibition of transforming growth factor-β signaling in diffuse-type gastric carcinoma. Cancer Sci. 100, 1809–1816 (2009).
Thiery, J. P. Epithelial-mesenchymal transitions in tumour progression. Nature Rev. Cancer 2, 442–454 (2002).
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Moustakas, A. & Heldin, C. H. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 98, 1512–1520 (2007). References 96, 97 and 98 cover in detail mechanisms of EMT and discuss the physiological and pathological roles of EMT.
Hurd, T. W., Gao, L., Roh, M. H., Macara, I. G. & Margolis, B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nature Cell Biol. 5, 137–142 (2003).
Ozdamar, B. et al. Regulation of the polarity protein Par6 by TGFβ receptors controls epithelial cell plasticity. Science 307, 1603–1609 (2005).
Xu, J., Lamouille, S. & Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 19, 156–172 (2009).
Oft, M. et al. TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 10, 2462–2477 (1996).
Janda, E. et al. Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 156, 299–313 (2002).
Vogelmann, R. et al. TGFβ-induced downregulation of E-cadherin-based cell-cell adhesion depends on PI3-kinase and PTEN. J. Cell Sci. 118, 4901–4912 (2005).
Horiguchi, K. et al. Role of Ras signaling in the induction of snail by transforming growth factor-β. J. Biol. Chem. 284, 245–253 (2009).
Araki, S. et al. TGF-β1-induced expression of human Mdm2 correlates with late-stage metastatic breast cancer. J. Clin. Invest. 120, 290–302 (2010).
Adorno, M. et al. A Mutant-p53/Smad complex opposes p63 to empower TGFβ-induced metastasis. Cell 137, 87–98 (2009).
Papageorgis, P. et al. Smad signaling is required to maintain epigenetic silencing during breast cancer progression. Cancer Res. 70, 968–978 (2010).
Zeisberg, M. et al. BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nature Med. 9, 964–968 (2003).
Buijs, J. T. et al. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res. 67, 8742–8751 (2007).
Buijs, J. T. et al. BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am. J. Pathol. 171, 1047–1057 (2007).
Saito, R. A. et al. Thyroid transcription factor-1 inhibits transforming growth factor-β-mediated epithelial-to-mesenchymal transition in lung adenocarcinoma cells. Cancer Res. 69, 2783–2791 (2009).
Li, C. et al. Transforming growth factor-β inhibits pulmonary surfactant protein B gene transcription through SMAD3 interactions with NKX2.1 and HNF-3 transcription factors. J. Biol. Chem. 277, 38399–38408 (2002).
Minoo, P. et al. SMAD3 prevents binding of NKX2.1 and FOXA1 to the SpB promoter through its MH1 and MH2 domains. Nucleic Acids Res. 36, 179–188 (2008).
Tan, D. et al. Thyroid transcription factor-1 expression prevalence and its clinical implications in non-small cell lung cancer: a high-throughput tissue microarray and immunohistochemistry study. Hum. Pathol. 34, 597–604 (2003).
Padua, D. et al. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133, 66–77 (2008). This paper demonstrates the role of TGFβ signalling in enabling the pulmonary metastasis of breast cancer cells.
Guise, T. A. et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J. Clin. Invest. 98, 1544–1549 (1996).
Yin, J. J. et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 103, 197–206 (1999).
Kondo, H., Guo, J. & Bringhurst, F. R. Cyclic adenosine monophosphate/protein kinase A mediates parathyroid hormone/parathyroid hormone-related protein receptor regulation of osteoclastogenesis and expression of RANKL and osteoprotegerin mRNAs by marrow stromal cells. J. Bone Miner. Res. 17, 1667–1679 (2002).
Kang, Y., Chen, C. R. & Massague, J. A self-enabling TGFβ response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol. Cell 11, 915–926 (2003).
Kingsley, L. A., Fournier, P. G., Chirgwin, J. M. & Guise, T. A. Molecular biology of bone metastasis. Mol. Cancer Ther. 6, 2609–2617 (2007).
Ehata, S. et al. Ki26894, a novel transforming growth factor-b type I receptor kinase inhibitor, inhibits in vitro invasion and in vivo bone metastasis of a human breast cancer cell line. Cancer Sci. 98, 127–133 (2007).
Bandyopadhyay, A. et al. Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-β type I receptor kinase inhibitor. Cancer Res. 66, 6714–6721 (2006).
Ge, R. et al. Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-β type I receptor kinase in vivo. Clin. Cancer Res. 12, 4315–4330 (2006).
Roberts, A. B. & Wakefield, L. M. The two faces of transforming growth factor β in carcinogenesis. Proc. Natl Acad. Sci. USA 100, 8621–8623 (2003).
Yingling, J. M., Blanchard, K. L. & Sawyer, J. S. Development of TGF-β signalling inhibitors for cancer therapy. Nature Rev. Drug Discov. 3, 1011–1022 (2004). This review covers various TGFβ inhibitors and discusses the rationale for evaluating them as cancer therapeutics.
Schlingensiepen, K. H. et al. Targeted tumor therapy with the TGF-β2 antisense compound AP 12009. Cytokine Growth Factor Rev. 17, 129–139 (2006).
Santamaria-Martínez, A. et al. Identification of multipotent mesenchymal stromal cells in the reactive stroma of a prostate cancer xenograft by side population analysis. Exp. Cell Res. 315, 3004–3013 (2009).
Kabashima, A. et al. Side population of pancreatic cancer cells predominates in TGF-b-mediated epithelial to mesenchymal transition and invasion. Int. J. Cancer. 124, 2771–2779 (2009).
Acknowledgements
We apologize to those colleagues whose publications could not be cited because of space limitations. Work from the authors' laboratory is supported by KAKENHI (Grant-in-Aid for Scientific Research) on Priority Areas 'New strategies for cancer therapy based on advancement of basic research' and the Global COE Program (Integrative Life Science Based on the Study of Biosignalling Mechanisms) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ikushima, H., Miyazono, K. TGFβ signalling: a complex web in cancer progression. Nat Rev Cancer 10, 415–424 (2010). https://doi.org/10.1038/nrc2853
Issue Date:
DOI: https://doi.org/10.1038/nrc2853
This article is cited by
-
Elucidating shared biomarkers and pathways in kidney stones and diabetes: insights into novel therapeutic targets and the role of resveratrol
Journal of Translational Medicine (2023)
-
Tumor–stroma ratio predicts prognosis and PD-L1 expression in hepatocellular carcinoma
BMC Cancer (2023)
-
Global serum profiling: an opportunity for earlier cancer detection
Journal of Experimental & Clinical Cancer Research (2023)
-
EMT/MET plasticity in cancer and Go-or-Grow decisions in quiescence: the two sides of the same coin?
Molecular Cancer (2023)
-
Invasive FoxM1 phosphorylated by PLK1 induces the polarization of tumor-associated macrophages to promote immune escape and metastasis, amplified by IFITM1
Journal of Experimental & Clinical Cancer Research (2023)