Abstract
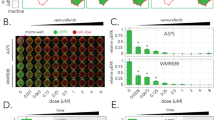
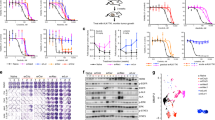
The principles that govern the evolution of tumors exposed to targeted therapy are poorly understood. Here we modeled the selection and propagation of an amplification in the BRAF oncogene (BRAFamp) in patient-derived tumor xenografts (PDXs) that were treated with a direct inhibitor of the kinase ERK, either alone or in combination with other ERK signaling inhibitors. Single-cell sequencing and multiplex fluorescence in situ hybridization analyses mapped the emergence of extra-chromosomal amplification in parallel evolutionary trajectories that arose in the same tumor shortly after treatment. The evolutionary selection of BRAFamp was determined by the fitness threshold, the barrier that subclonal populations need to overcome to regain fitness in the presence of therapy. This differed for inhibitors of ERK signaling, suggesting that sequential monotherapy is ineffective and selects for a progressively higher BRAF copy number. Concurrent targeting of the RAF, MEK and ERK kinases, however, imposed a sufficiently high fitness threshold to prevent the propagation of subclones with high-level BRAFamp. When administered on an intermittent schedule, this treatment inhibited tumor growth in 11/11 PDXs of lung cancer or melanoma without apparent toxicity in mice. Thus, gene amplification can be acquired and expanded through parallel evolution, enabling tumors to adapt while maintaining their intratumoral heterogeneity. Treatments that impose the highest fitness threshold will likely prevent the evolution of resistance-causing alterations and, thus, merit testing in patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 511, 543–550 (2014).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002).
Flaherty, K.T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010).
Planchard, D. et al. Dabrafenib in patients with BRAFV600E-positive advanced non-small-cell lung cancer: a single-arm, multicenter, open-label, phase 2 trial. Lancet Oncol. 17, 642–650 (2016).
Flaherty, K.T. et al. Combined BRAF and MEK inhibition in melanoma with BRAFV600 mutations. N. Engl. J. Med. 367, 1694–1703 (2012).
Larkin, J. et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 371, 1867–1876 (2014).
Long, G.V. et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 371, 1877–1888 (2014).
Planchard, D. et al. Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, multicenter phase 2 trial. Lancet Oncol. 17, 984–993 (2016).
Poulikakos, P.I. et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAFV600E. Nature 480, 387–390 (2011).
Shi, H. et al. Melanoma whole-exome sequencing identifies V600EB-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat. Commun. 3, 724 (2012).
Das Thakur, M. et al. Modeling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 494, 251–255 (2013).
Shi, H. et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 4, 80–93 (2014).
Lito, P., Rosen, N. & Solit, D.B. Tumor adaptation and resistance to RAF inhibitors. Nat. Med. 19, 1401–1409 (2013).
Bollag, G. et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467, 596–599 (2010).
Lito, P. et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell 22, 668–682 (2012).
Lipinski, K.A. et al. Cancer evolution and the limits of predictability in precision cancer medicine. Trends Cancer 2, 49–63 (2016).
McGranahan, N. & Swanton, C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 27, 15–26 (2015).
Merlo, L.M., Pepper, J.W., Reid, B.J. & Maley, C.C. Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 6, 924–935 (2006).
Baslan, T. et al. Genome-wide copy number analysis of single cells. Nat. Protoc. 7, 1024–1041 (2012).
Garvin, T. et al. Interactive analysis and assessment of single-cell copy number variations. Nat. Methods 12, 1058–1060 (2015).
Navin, N. et al. Tumor evolution inferred by single-cell sequencing. Nature 472, 90–94 (2011).
Morris, E.J. et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov. 3, 742–750 (2013).
Wong, D.J. et al. Antitumor activity of the ERK inhibitor SCH772984 against BRAF-mutant, NRAS-mutant and wild-type melanoma. Mol. Cancer 13, 194 (2014).
Baslan, T. et al. Optimizing sparse sequencing of single cells for highly multiplex copy number profiling. Genome Res. 25, 714–724 (2015).
Weir, B.A. et al. Characterizing the cancer genome in lung adenocarcinoma. Nature 450, 893–898 (2007).
Yao, Z. et al. BRAF mutants evade ERK-dependent feedback by different mechanisms that determine their sensitivity to pharmacologic inhibition. Cancer Cell 28, 370–383 (2015).
Joseph, E.W. et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF–selective manner. Proc. Natl. Acad. Sci. USA 107, 14903–14908 (2010).
Kim, K.B. et al. Phase 2 study of the MEK1 and MEK2 inhibitor trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J. Clin. Oncol. 31, 482–489 (2013).
Corcoran, R.B. et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF-mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2, 227–235 (2012).
Prahallad, A. et al. Unresponsiveness of colon cancer to BRAFV600E inhibition through feedback activation of EGFR. Nature 483, 100–103 (2012).
Shi, H. et al. A novel AKT1 mutant amplifies an adaptive melanoma response to BRAF inhibition. Cancer Discov. 4, 69–79 (2014).
Sun, C. et al. Reversible and adaptive resistance to BRAFV600E inhibition in melanoma. Nature 508, 118–122 (2014).
Maertens, O. et al. Elucidating distinct roles for NF1 in melanomagenesis. Cancer Discov. 3, 338–349 (2013).
Johannessen, C.M. et al. A melanocyte lineage program confers resistance to MAP kinase pathway inhibition. Nature 504, 138–142 (2013).
Anderson, K. et al. Genetic variegation of clonal architecture and propagating cells in leukemia. Nature 469, 356–361 (2011).
Melchor, L. et al. Single-cell genetic analysis reveals the composition of initiating clones and phylogenetic patterns of branching and parallel evolution in myeloma. Leukemia 28, 1705–1715 (2014).
Campbell, P.J. et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 467, 1109–1113 (2010).
Nguyen, A. et al. PKLR promotes colorectal cancer liver colonization through induction of glutathione synthesis. J. Clin. Invest. 126, 681–694 (2016).
Poirier, J.T. et al. DNA methylation in small-cell lung cancer defines distinct disease subtypes and correlates with high expression of EZH2. Oncogene 34, 5869–5878 (2015).
Einarsdottir, B.O. et al. Melanoma-patient-derived xenografts accurately model the disease and develop fast enough to guide treatment decisions. Oncotarget 5, 9609–9618 (2014).
Cheng, D.T. et al. Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization-capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 17, 251–264 (2015).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Li, H. & Durbin, R. Fast and accurate short-read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Gao, R. et al. Punctuated copy number evolution and clonal stasis in triple-negative breast cancer. Nat. Genet. 48, 1119–1130 (2016).
Wanjala, J. et al. Identifying actionable targets through integrative analyses of GEM model and human prostate cancer genomic profiling. Mol. Cancer Ther. 14, 278–288 (2015).
Hembrough, T. et al. Application of selected reaction monitoring for multiplex quantification of clinically validated biomarkers in formalin-fixed, paraffin-embedded tumor tissue. J. Mol. Diagn. 15, 454–465 (2013).
Catenacci, D.V. et al. Absolute quantitation of Met using mass spectrometry for clinical application: assay precision, stability and correlation with MET gene amplification in FFPE tumor tissue. PLoS One 9, e100586 (2014).
Lito, P., Solomon, M., Li, L.S., Hansen, R. & Rosen, N. Allele-specific inhibitors inactivate mutant KRASG12C by a trapping mechanism. Science 351, 604–608 (2016).
Acknowledgements
The authors thank D. Solit, N. Bouvier and the Center for Molecular Oncology at MSKCC for assistance with next-generation sequencing, Z. Yao (MSKCC) for providing the A375 doxycycline-inducible BRAFV600E cells, as well as C. Sawyers, C. Rudin, J. Poirier and M. Mroczkowski for reviewing the manuscript. This work was supported by the US National Institutes of Health (NIH) (grant K08 CA191082-01A1; P.L.), the Uniting Against Lung Cancer Foundation (P.L.), the Damon Runyon Clinical Investigator Award (P.L.), the Josie Robertson Investigator Program at MSKCC (P.L.), the Druckenmiller Center for Lung Cancer Center at MSKCC (P.L.) and a Medical Scientist Training Program grant from the National Institute of General Medical Sciences of the NIH under award number T32GM007739 to the Weill Cornell–Rockefeller–Sloan Kettering Tri-institutional MD–PhD Program (Y.X.). E.d.S. and S.W.L. are supported in part by the MSKCC Pilot Center for Precision Disease Modeling program (U54 OD020355). T.B. is supported by the William C. and Joyce C. O'Neil Charitable Trust, Memorial Sloan Kettering Single-Cell Sequencing Initiative. J.N. is supported by the Knut and Alice Wallenberg Foundation. The authors also acknowledge the MSKCC Support Grant–Core Grant program (P30 CA008748).
Author information
Authors and Affiliations
Contributions
Y.X., L.M., A.V., M.S., T.T.M. and N.C. performed experiments; L.M., T.B. and J.S.R.-F. performed, analyzed and helped interpret the single-cell sequencing experiments; M.F.B. analyzed the bulk-sequencing data; G.J.R. and B.T.L. provided the patient samples; J.N. and U.S. provided the PDX models; E.d.S. performed the animal experiments; K.S., F.C., T.H. and S.S. performed the mass spectrometry experiments; K.C. performed the FISH experiments, and G.N. analyzed the results. N.R. and S.W.L. provided key scientific insights and reagents; P.L. conceived and supervised the study, designed and performed experiments, and interpreted data; Y.X. and P.L. were the principal writers of the manuscript; and all of the authors reviewed the manuscript and contributed in writing.
Corresponding author
Ethics declarations
Competing interests
P.L. is listed as an inventor on a patent application filed by MSKCC that incorporates discoveries described in the manuscript. N.R. is on the scientific advisory board, and has received grant support from, Chugai Pharmaceutical and is on the SAB of Astra-Zeneca, Beigene and Kura. S.S., T.H., K.S. and F.C. are employees of NantOmics.
Supplementary information
Supplementary Tables and Figures
Supplementary Tables 1 and 2 and Supplementary Figures 1–7 (PDF 11679 kb)
Rights and permissions
About this article
Cite this article
Xue, Y., Martelotto, L., Baslan, T. et al. An approach to suppress the evolution of resistance in BRAFV600E-mutant cancer. Nat Med 23, 929–937 (2017). https://doi.org/10.1038/nm.4369
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4369
This article is cited by
-
BRAF — a tumour-agnostic drug target with lineage-specific dependencies
Nature Reviews Clinical Oncology (2024)
-
ERK pathway agonism for cancer therapy: evidence, insights, and a target discovery framework
npj Precision Oncology (2024)
-
Epithelial-to-mesenchymal transition in cancer progression: unraveling the immunosuppressive module driving therapy resistance
Cancer and Metastasis Reviews (2024)
-
DNA replication stress and mitotic catastrophe mediate sotorasib addiction in KRASG12C-mutant cancer
Journal of Biomedical Science (2023)
-
Extrachromosomal circular DNA in colorectal cancer: biogenesis, function and potential as therapeutic target
Oncogene (2023)