Abstract
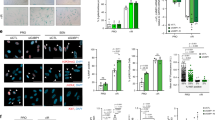
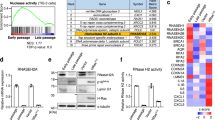
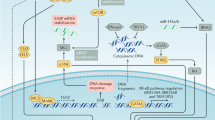
Oncogene-induced senescence (OIS) is crucial for tumour suppression. Senescent cells implement a complex pro-inflammatory response termed the senescence-associated secretory phenotype (SASP). The SASP reinforces senescence, activates immune surveillance and paradoxically also has pro-tumorigenic properties. Here, we present evidence that the SASP can also induce paracrine senescence in normal cells both in culture and in human and mouse models of OIS in vivo. Coupling quantitative proteomics with small-molecule screens, we identified multiple SASP components mediating paracrine senescence, including TGF-β family ligands, VEGF, CCL2 and CCL20. Amongst them, TGF-β ligands play a major role by regulating p15INK4b and p21CIP1. Expression of the SASP is controlled by inflammasome-mediated IL-1 signalling. The inflammasome and IL-1 signalling are activated in senescent cells and IL-1α expression can reproduce SASP activation, resulting in senescence. Our results demonstrate that the SASP can cause paracrine senescence and impact on tumour suppression and senescence in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Kuilman, T., Michaloglou, C., Mooi, W. J. & Peeper, D. S. The essence of senescence. Genes Dev. 24, 2463–2479 (2010).
Campisi, J. & d’Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 (2007).
Kuilman, T. & Peeper, D.S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer 9, 81–94 (2009).
Coppe, J. P. et al. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One 5, e9188 (2010).
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001).
Coppe, J. P., Kauser, K., Campisi, J. & Beausejour, C. M. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 281, 29568–29574 (2006).
Coppe, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Acosta, J. C. et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 (2008).
Kuilman, T. et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008).
Kortlever, R. M., Higgins, P. J. & Bernards, R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 8, 877–884 (2006).
Wajapeyee, N., Serra, R. W., Zhu, X., Mahalingam, M. & Green, M. R. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132, 363–374 (2008).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008).
Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 (1965).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Nelson, G. et al. A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11, 345–349 (2012).
Fridman, A. L. & Tainsky, M. A. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene 27, 5975–5987 (2008).
Collado, M. et al. Tumour biology: senescence in premalignant tumours. Nature 436, 642 (2005).
Massague, J. TGFbeta in Cancer. Cell 134, 215–230 (2008).
Kaneda, A. et al. Activation of Bmp2-Smad1 signal and its regulation by coordinated alteration of H3K27 trimethylation in Ras-induced senescence. PLoS Genet. 7, e1002359 (2011).
Reynisdottir, I., Polyak, K., Iavarone, A. & Massague, J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF- β. Genes Dev. 9, 1831–1845 (1995).
Hingorani, S. R. et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, 437–450 (2003).
Morton, J. P. et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc. Natl Acad. Sci. USA 107, 246–251 (2010).
Larsson, J. et al. Abnormal angiogenesis but intact hematopoietic potential in TGF- β type I receptor-deficient mice. EMBO J. 20, 1663–1673 (2001).
Morton, J. P. et al. LKB1 haploinsufficiency cooperates with Kras to promote pancreatic cancer through suppression of p21-dependent growth arrest. Gastroenterology 139, 586–597 (2010).
Morton, J. P. et al. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 139, 292–303 (2010).
Orjalo, A. V., Bhaumik, D., Gengler, B. K., Scott, G. K. & Campisi, J. Cell surface-bound IL-1α is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl Acad. Sci. USA 106, 17031–17036 (2009).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Strowig, T., Henao-Mejia, J., Elinav, E. & Flavell, R. Inflammasomes in health and disease. Nature 481, 278–286 (2012).
Carragher, L. A. et al. V600EBraf induces gastrointestinal crypt senescence and promotes tumour progression through enhanced CpG methylation of p16INK4a. EMBO Mol. Med. 2, 458–471 (2010).
Keller, M., Ruegg, A., Werner, S. & Beer, H. D. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 (2008).
Sibilia, M. et al. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell 102, 211–220 (2000).
Kriegl, L. et al. Up and downregulation of p16(Ink4a) expression in BRAF-mutated polyps/adenomas indicates a senescence barrier in the serrated route to colon cancer. Mod. Pathol. 24, 1015–1022 (2011).
Bennecke, M. et al. Ink4a/Arf and oncogene-induced senescence prevent tumor progression during alternative colorectal tumorigenesis. Cancer Cell 18, 135–146 (2010).
Caruso, M. et al. Over-expression of cathepsin E and trefoil factor 1 in sessile serrated adenomas of the colorectum identified by gene expression analysis. Virchows Archiv. 454, 291–302 (2009).
Schmitt, C. A. Cellular senescence and cancer treatment. Biochim. Biophys. Acta 1775, 5–20 (2007).
Prise, K. M. & O’Sullivan, J. M. Radiation-induced bystander signalling in cancer therapy. Nature Rev. Cancer 9, 351–360 (2009).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Burd, C. E. et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell 152, 340–351 (2013).
Chien, Y. et al. Control of the senescence-associated secretory phenotype by NF-{κ}B promotes senescence and enhances chemosensitivity. Genes Dev. 25, 2125–2136 (2011).
Jing, H. et al. Opposing roles of NF-{κ}B in anti-cancer treatment outcome unveiled by cross-species investigations. Genes Dev. 25, 2137–2146 (2011).
Zitvogel, L., Kepp, O., Galluzzi, L. & Kroemer, G. Inflammasomes incarcinogenesis and anticancer immune responses. Nat. Immun. 13, 343–351 (2012).
Ling, J. et al. KrasG12D-induced IKK2/β/NF-κB activation by IL-1α and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell 21, 105–120 (2012).
Barradas, M. et al. Histone demethylase JMJD3 contributes to epigenetic control of INK4a/ARF by oncogenic RAS. Genes Dev. 23, 1177–1182 (2009).
Banito, A. & Gil, J. Induced pluripotent stem cells and senescence: learning the biology to improve the technology. EMBO Rep. 11, 353–359 (2010).
Banito, A. et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev 23, 2134–2139 (2009).
Bishop, C.L. et al. Primary cilium-dependent and -independent Hedgehog signaling inhibits p16(INK4A). Mol. Cell 40, 533–547 (2010).
Acosta, J. C., Snijders, A. P. & Gil, J. Unbiased characterization of the senescence-associated secretome using SILAC-based quantitative proteomics. Methods Mol. Biol. 965, 175–184 (2013).
Chen, Y. et al. SPD–a web-based secreted protein database. Nucleic Acids Res. 33, D169–D73 (2005).
Gonzalez, R. et al. Screening the mammalian extracellular proteome for regulators of embryonic human stem cell pluripotency. Proc. Natl Acad. Sci. USA 107, 3552–3557 (2010).
Jackson, E. L. et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 15, 3243–3248 (2001).
Capper, D. et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 122, 11–19 (2011).
Acknowledgements
We are grateful to M. Stampfer (Lawrence Berkeley National Laboratory, USA), G. Núñez (University of Michigan, USA) and D. Escors (UCL, UK) for reagents and to T. Bird, S. Forbes, V. Episkopou, T. Rodrı´guez, P. Schirmacher, S. Parrinello, M. Narita, G. Peters and D. Beach for advice and critical reading of the manuscript. We also thank the tissue bank of the National Center for Tumour Diseases Heidelberg for providing colon tissues. Core support from the MRC and grants from MRCT, CRUK and the AICR financially supported the research in J.G’s laboratory. J.G. is also supported by the EMBO Young Investigator Programme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2745 kb)
Supplementary Table 1
Supplementary Information (XLS 9882 kb)
Supplementary Table 2
Supplementary Information (XLS 294 kb)
Supplementary Table 3
Supplementary Information (XLS 41 kb)
Supplementary Table 4
Supplementary Information (XLS 29 kb)
Supplementary Table 5
Supplementary Information (XLS 25 kb)
Supplementary Table 6
Supplementary Information (XLS 26 kb)
Supplementary Table 7
Supplementary Information (XLS 27 kb)
Supplementary Table 8
Supplementary Information (XLS 174 kb)
Rights and permissions
About this article
Cite this article
Acosta, J., Banito, A., Wuestefeld, T. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15, 978–990 (2013). https://doi.org/10.1038/ncb2784
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2784
This article is cited by
-
Modulation of fracture healing by senescence-associated secretory phenotype (SASP): a narrative review of the current literature
European Journal of Medical Research (2024)
-
Fisetin, a potential skin rejuvenation drug that eliminates senescent cells in the dermis
Biogerontology (2024)
-
Targeting therapy-induced senescence as a novel strategy to combat chemotherapy-induced peripheral neuropathy
Supportive Care in Cancer (2024)
-
Senolytic treatment does not mitigate oxidative stress-induced muscle atrophy but improves muscle force generation in CuZn superoxide dismutase knockout mice
GeroScience (2024)
-
Connexin-43 hemichannels orchestrate NOD-like receptor protein-3 (NLRP3) inflammasome activation and sterile inflammation in tubular injury
Cell Communication and Signaling (2023)