Abstract
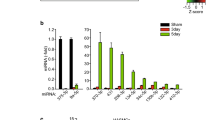
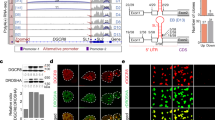
MicroRNAs (miRNAs) are small non-coding RNAs that participate in the spatiotemporal regulation of messenger RNA and protein synthesis. Aberrant miRNA expression leads to developmental abnormalities and diseases, such as cardiovascular disorders and cancer; however, the stimuli and processes regulating miRNA biogenesis are largely unknown. The transforming growth factor β (TGF-β) and bone morphogenetic protein (BMP) family of growth factors orchestrates fundamental biological processes in development and in the homeostasis of adult tissues, including the vasculature. Here we show that induction of a contractile phenotype in human vascular smooth muscle cells by TGF-β and BMPs is mediated by miR-21. miR-21 downregulates PDCD4 (programmed cell death 4), which in turn acts as a negative regulator of smooth muscle contractile genes. Surprisingly, TGF-β and BMP signalling promotes a rapid increase in expression of mature miR-21 through a post-transcriptional step, promoting the processing of primary transcripts of miR-21 (pri-miR-21) into precursor miR-21 (pre-miR-21) by the DROSHA (also known as RNASEN) complex. TGF-β- and BMP-specific SMAD signal transducers are recruited to pri-miR-21 in a complex with the RNA helicase p68 (also known as DDX5), a component of the DROSHA microprocessor complex. The shared cofactor SMAD4 is not required for this process. Thus, regulation of miRNA biogenesis by ligand-specific SMAD proteins is critical for control of the vascular smooth muscle cell phenotype and potentially for SMAD4-independent responses mediated by the TGF-β and BMP signalling pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
21 July 2008
In the online-only extended Methods, the miR-214 primer sequence was corrected on 21st July 2008
References
ten Dijke, P. & Arthur, H. M. Extracellular control of TGFβ signalling in vascular development and disease. Nature Rev. Mol. Cell Biol. 8, 857–868 (2007)
Morrell, N. W. Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling? Proc. Am. Thorac. Soc. 3, 680–686 (2006)
Owens, G. K. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 75, 487–517 (1995)
Rensen, S. S. M., Doevendans, P. A. F. M. & van Eys, G. J. J. M. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Netherlands Heart J. 15, 100–108 (2007)
Lagna, G. et al. Control of phenotypic plasticity of smooth muscle cells by BMP signaling through the myocardin-related transcription factors. J. Biol. Chem. 282, 37244–37255 (2007)
Owens, G. K., Kumar, M. S. & Wamhoff, B. R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84, 767–801 (2004)
Ji, R. et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of microRNA in vascular neointimal lesion formation. Circ. Res. 100, 1579–1588 (2007)
Esau, C. C. Inhibition of microRNA with antisense oligonucleotides. Methods 44, 55–60 (2008)
van Rooij, E. et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl Acad. Sci. USA 103, 18255–18260 (2006)
Asangani, I. A. et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27, 2128–2136 (2008)
Frankel, L. B. et al. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J. Biol. Chem. 283, 1026–1033 (2007)
Zhu, S. et al. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 18, 350–359 (2008)
Hollnagel, A., Oehlmann, V., Heymer, J., Ruther, U. & Nordheim, A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J. Biol. Chem. 274, 19838–19845 (1999)
Chan, M. C. et al. A novel regulatory mechanism of the bone morphogenetic protein (BMP) signaling pathway involving the carboxyl-terminal tail domain of BMP type II receptor. Mol. Cell. Biol. 27, 5776–5789 (2007)
Lee, Y. et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415–419 (2003)
Han, J. et al. The Drosha–DGCR8 complex in primary microRNA processing. Genes Dev. 18, 3016–3027 (2004)
Landthaler, M., Yalcin, A. & Tuschl, T. The human DiGeorge syndrome critical region gene 8 and its D. melanogaster homolog are required for miRNA biogenesis. Curr. Biol. 14, 2162–2167 (2004)
Fukuda, T. et al. DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nature Cell Biol. 9, 604–611 (2007)
Kim, V. N. & Nam, J. W. Genomics of microRNA. Trends Genet. 22, 165–173 (2006)
Kim, V. N. MicroRNA biogenesis: coordinated cropping and dicing. Nature Rev. Mol. Cell Biol. 6, 376–385 (2005)
Zhao, Y. & Srivastava, D. A developmental view of microRNA function. Trends Biochem. Sci. 32, 189–197 (2007)
Lee, E. J. et al. Systematic evaluation of microRNA processing patterns in tissues, cell lines, and tumors. RNA 14, 35–42 (2007)
Obernosterer, G., Leuschner, P. J., Alenius, M. & Martinez, J. Post-transcriptional regulation of microRNA expression. RNA 12, 1161–1167 (2006)
Thomson, J. M. et al. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 20, 2202–2207 (2006)
Wulczyn, F. G. et al. Post-transcriptional regulation of the let-7 microRNA during neural cell specification. FASEB J. 21, 415–426 (2007)
Guil, S. & Caceres, J. F. The multifunctional RNA-binding protein hnRNP A1 is required for processing of miR-18a. Nature Struct. Mol. Biol. 14, 591–596 (2007)
Massague, J., Seoane, J. & Wotton, D. Smad transcription factors. Genes Dev. 19, 2783–2810 (2005)
Schmierer, B. & Hill, C. S. TGFβ–SMAD signal transduction: molecular specificity and functional flexibility. Nature Rev. Mol. Cell Biol. 8, 970–982 (2007)
Schmittgen, T. D. et al. Real-time PCR quantification of precursor and mature microRNA. Methods 44, 31–38 (2008)
Korchynskyi, O. & ten Dijke, P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem. 277, 4883–4891 (2002)
Loffler, D. et al. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood 110, 1330–1333 (2007)
Zhu, S., Si, M. L., Wu, H. & Mo, Y. Y. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J. Biol. Chem. 282, 14328–14336 (2007)
Warner, D. R. et al. Functional interaction between Smad, CREB binding protein, and p68 RNA helicase. Biochem. Biophys. Res. Commun. 324, 70–76 (2004)
Kretzschmar, M., Liu, F., Hata, A., Doody, J. & Massagué, J. The TGF-β mediator Smad1 is directly phosphorylated and functionally activated by the BMP receptor kinase. Genes Dev. 11, 984–995 (1997)
Gomis, R. R. et al. A FoxO–Smad synexpression group in human keratinocytes. Proc. Natl Acad. Sci. USA 103, 12747–12752 (2006)
Levy, L. & Hill, C. S. Smad4 dependency defines two classes of transforming growth factor β (TGF-β) target genes and distinguishes TGF-β-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol. Cell. Biol. 25, 8108–8125 (2005)
Giehl, K., Imamichi, Y. & Menke, A. Smad4-independent TGF-β signaling in tumor cell migration. Cells Tissues Organs 185, 123–130 (2007)
Ijichi, H. et al. Smad4-independent regulation of p21/WAF1 by transforming growth factor-β. Oncogene 23, 1043–1051 (2004)
Si, M. L. et al. miR-21-mediated tumor growth. Oncogene 26, 2799–2803 (2007)
Diederichs, S. & Haber, D. A. Sequence variations of microRNAs in human cancer: alterations in predicted secondary structure do not affect processing. Cancer Res. 66, 6097–6104 (2006)
Volinia, S. et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl Acad. Sci. USA 103, 2257–2261 (2006)
Iorio, M. V. et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 65, 7065–7070 (2005)
Wiemer, E. A. The role of microRNAs in cancer: no small matter. Eur. J. Cancer 43, 1529–1544 (2007)
Bierie, B. & Moses, H. L. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nature Rev. Cancer 6, 506–520 (2006)
Arteaga, C. L. Inhibition of TGFβ signaling in cancer therapy. Curr. Opin. Genet. Dev. 16, 30–37 (2006)
Bachman, K. E. & Park, B. H. Duel nature of TGF-β signaling: tumor suppressor vs. tumor promoter. Curr. Opin. Oncol. 17, 49–54 (2005)
Glick, A. B. TGFβ1, back to the future: revisiting its role as a transforming growth factor. Cancer Biol. Ther. 3, 276–283 (2004)
Massague, J. & Gomis, R. R. The logic of TGFβ signaling. FEBS Lett. 580, 2811–2820 (2006)
Fujii, M. et al. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol. Biol. Cell 10, 3801–3813 (1999)
Shi, Y. et al. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA-binding in TGFβ signaling. Cell 94, 585–594 (1998)
Acknowledgements
We thank M.-C. Chan and N. Neuman for critical discussion and H. Surks and M. Ivan for critical reading of the manuscript and technical advice. We also thank S. Kato, E. Olson, R. Bassel-Duby, Y.Y. Mo, K. Miyazono, B. Cochran and G.-R. Wang for sharing reagents. This work was supported by grants from the National Institute of Health HD042149 and HL082854 to A.H. and HL086572 to G.L.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures
The file contains Supplementary Figures 1-21 with Legends. (PDF 13651 kb)
Rights and permissions
About this article
Cite this article
Davis, B., Hilyard, A., Lagna, G. et al. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454, 56–61 (2008). https://doi.org/10.1038/nature07086
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07086
This article is cited by
-
Ribosomal protein L24 mediates mammalian microRNA processing in an evolutionarily conserved manner
Cellular and Molecular Life Sciences (2024)
-
Multi-omics in thoracic aortic aneurysm: the complex road to the simplification
Cell & Bioscience (2023)
-
Nuclear receptor RXRα binds the precursor of miR-103 to inhibit its maturation
BMC Biology (2023)
-
Cellular functions of eukaryotic RNA helicases and their links to human diseases
Nature Reviews Molecular Cell Biology (2023)
-
Interplay Between TGF-β Signaling and MicroRNA in Diabetic Cardiomyopathy
Cardiovascular Drugs and Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.