Abstract
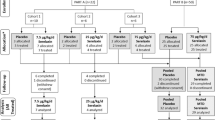
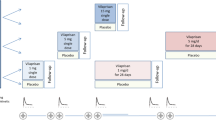
The pharmacokinetics of recombinant human relaxin (rhRlx) after intravenous (iv) bolus administration and the absorption of rhRlx after intracervical or intravaginal administration were determined in nonpregnant women. The study was conducted in two parts. In part I, 25 women received 0.01 mg/kg rhRlx iv. After a minimum 7-day washout period, these women were dosed intracervically (n = 10) or intravaginally (n = 15) with 0.75 or 1.5 mg rhRlx, respectively, in 3% methylcellulose gel. Part II was a double-blind, randomized, three-way crossover study in 26 women. At 1-month intervals, each woman received one of three intravaginal treatments consisting of 0 (placebo), 1, or 6 mg rhRlx in 3% methylcellulose gel. The serum concentrations of relaxin following iv administration were described as the sum of three exponentials. The mean (±SD) initial, intermediate, and terminal half-lives were 0.09 ± 0.04, 0.72 ± 0.11, and 4.6 ± 1.2 hr, respectively. Most of the area under the curve was associated with the intermediate half-life. The weight-normalized clearance was 170 ± 50 mL/hr/kg. The observed peak concentration was 98 ± 29 ng/mL, and the weight-normalized initial volume of distribution was 78 ± 40 mL/kg, which is approximately equivalent to the serum volume. If central compartment elimination was assumed, the volume of distribution at steady state (V ss/W) was 280 ± 100 mL/kg, which is approximately equivalent to extracellular fluid volume. V ss/W could be as large as 1300 ± 400 mL/kg without this assumption. After intravaginal administration of the placebo gel, endogenous relaxin concentrations were evident (i.e., ≥20 pg/mL) in 9 of the 26 women (maximum concentrations, 23–234 pg/mL). A similar proportion of women (approximately 35–40%) exhibited measurable serum concentrations of relaxin following intravaginal rhRlx treatment; this proportion increased to 90% following intracervical rhRlx treatment. For both routes of administration, the maximum serum concentrations of relaxin were usually within the range of values observed for endogenous relaxin, suggesting that the absorption of rhRlx was minimal.
Similar content being viewed by others
REFERENCES
O. D. Sherwood. Relaxin. In E. Knobil and J. Neill (eds.), The Physiology of Reproduction, Raven Press, New York, 1988, pp. 585–673.
F. S. Khan-Dawood, L. T. Goldsmith, G. Weiss, and M. Y. Dawood. Human corpus luteum secretion of relaxin, oxytocin, and progesterone. J. Clin. Endocrinol. Metab. 68:627–631 (1989).
P. Hudson, M. John, R. Crawford, J. Haralambidis, D. Scanlon, J. Gorman, G. Tregear, J. Shine, and H. Niall. Relaxin gene expression in human ovaries and the predicted structure of a human preprorelaxin by analysis of cDNA clones. EMBO J. 4:2333–2339 (1984).
V. Sakbun, S. M. Ali, F. C. Greenwood, and G. D. Bryant-Greenwood. Human relaxin in the amnion, chorion, decidua parietalis, basal plate, and placental trophoblast by immunohistochemistry and northern analysis. J. Clin. Endocrinol. Metab. 70:508–514 (1990).
D. R. Stewart, A. C. Celniker, C. A. Taylor, J. R. Cragnun, J. W. Overstreet, and B. L. Lasley. Relaxin in the peri-implantation period. J. Clin. Endocrinol. Metab. 70:1771–1773 (1990).
M. R. Johnson, M. E. Wren, H. Abdalla, A. Kirkland, A. C. J. Allman, and S. L. Lightman. Relaxin levels in ovum donation pregnancies. Fertil. Steril. 56:59–61 (1991).
L. W. Eddie, I. T. Cameron, J. F. Leeton, D. L. Healy, and P. Renou. Ovarian relaxin is not essential for dilation of cervix. Lancet 336:243 (1990).
A. H. MacLennan, R. C. Green, G. D. Bryant-Greenwood, F. C. Greenwood, and R. F. Seamark. Ripening of the human cervix and induction of labour with purified porcine relaxin. Lancet 1:220–223 (1980).
A. H. MacLennan, R. C. Green, G. D. Bryant-Greenwood, F. C. Greenwood, and R. F. Seamark. Cervical ripening with combinations of vaginal prostaglandin F2a, estradiol, and relaxin. Obstet. Gynecol. 58:601–604 (1981).
S. J. Shire, L. A. Holladay, and E. Rinderknecht. Self-association of human and porcine relaxin as assessed by analytical ultracentrifugation and circular dichroism. Biochemistry 30:7703–7711 (1991).
A. H. MacLennan, R. C. Green, P. Grant, and R. Nicolson. Ripening of the human cervix and induction of labor with intracervical purified porcine relaxin. Obstet. Gynecol. 68:598–601 (1986).
D. P. Benziger and J. Edelson. Absorption from the vagina. Drug Metab. Rev. 14:137–168 (1983).
E. Canova-Davis, T. J. Kessler, P.-J. Lee, D. T. W. Fei, P. Griffin, J. T. Stults, J. D. Wade, and E. Rinderknecht. Use of recombinant DNA derived human relaxin to probe the structure of the native protein. Biochemistry 30:6006–6013 (1991).
J. W. Winslow, A. Shih, J. H. Bourell, G. Weiss, B. Reed, J. T. Stults, and L. T. Goldsmith. Human seminal relaxin is a product of the same gene as human luteal relaxin. Endocrinology 130:2660–2668 (1992).
B. L. Ferraiolo, J. Winslow, G. Laramee, A. Celniker, and P. Johnston. The pharmacokinetics and metabolism of human relaxins in rhesus monkeys. Pharm. Res. 8:1032–1038 (1991).
M. Rånby, N. Bergsdorf, T. Nilsson, G. Mellbring, B. Winblad, and G. Bucht. Age dependence of tissue plasminogen activator concentrations in plasma, as studied by an improved enzyme-linked immunosorbent assay. Clin. Chem. 32:2160–2165 (1986).
J. G. Wagner. Linear pharmacokinetic equations allowing direct calculation of many needed pharmacokinetic parameters from the coefficients and exponents of polyexponential equations which have been fitted to the data. J. Pharmacokin. Biopharm. 4:443–467 (1976).
J. Mordenti and A. Rescigno. Estimation of permanence time, exit time, dilution factor, and steady-state volume of distribution. Pharm. Res. 9:17–25 (1992).
P. A. Cossum, K. A. Dwyer, M. Roth, S. A. Chen, B. Moffat, R. Vandlen, and B. L. Ferraiolo. The disposition of a human relaxin (hRlx-2) in pregnant and nonpregnant rats. Pharm. Res. 9:419–424 (1992).
M. Rowland and T. N. Tozer. In Clinical Pharmacokinetics: Concepts and Applications, Lea and Febiger, Philadelphia, 1980, pp. 48–78.
J. W. Prothero. Scaling of blood parameters in mammals. Comp. Biochem. Physiol. 67A:649–657 (1980).
H. Ludwig and H. Metzger. The Human Female Reproduction Tract, Springer-Verlag, Berlin, 1976, pp. 8–15.
J. Mordenti, S. A. Chen, J. A. Moore, B. L. Ferraiolo, and J. D. Green. Interspecies scaling of clearance and volume of distribution data for five therapeutic proteins. Pharm. Res. 8:1351–1359 (1991).
S. A. Chen, B. Reed, T. Nguyen, N. Gaylord, G. B. Fuller, and J. Mordenti. The pharmacokinetics and absorption of recombinant human relaxin in nonpregnant rabbits and rhesus monkeys after intravenous and intravaginal administration. Pharm. Res. 10:223–227 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, S.A., Perlman, A.J., Spanski, N. et al. The Pharmacokinetics of Recombinant Human Relaxin in Nonpregnant Women After Intravenous, Intravaginal, and Intracervical Administration. Pharm Res 10, 834–838 (1993). https://doi.org/10.1023/A:1018901009062
Issue Date:
DOI: https://doi.org/10.1023/A:1018901009062