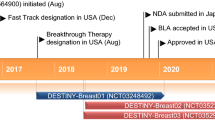
Abstract
Neratinib (Nerlynx™) is an oral, irreversible inhibitor of the human epidermal growth factor receptors HER1 (EGFR), HER2 and HER4. The drug originally arose from research by Wyeth (now Pfizer) and is now being developed by Puma Biotechnology primarily for the treatment of HER2-positive (HER+) breast cancer. Neratinib is approved in the USA for the extended adjuvant treatment of patients with HER2+ early-stage breast cancer who have been previously treated with a trastuzumab-based adjuvant regimen, and is in the preregistration phase for this indication in the EU. Neratinib, as monotherapy and/or combination therapy, is also in phase 3 development for metastatic breast cancer and in phase 1/2 development for advanced breast cancer and other solid tumours, including non-small cell lung cancer, colorectal cancer and glioblastoma. This article summarizes the milestones in the development of neratinib leading to this first approval for breast cancer.
Similar content being viewed by others
References
Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748.
Tai W, Mahato R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Control Release. 2010;146(3):264–75.
Wang T, Xu Y, Sheng S, et al. HER2 somatic mutations are associated with poor survival in HER2-negative breast cancers. Cancer Sci. 2017;108(4):671–7.
Schroeder RL, Stevens CL, Sridhar J. Small molecule tyrosine kinase inhibitors of ErbB2/HER2/Neu in the treatment of aggressive breast cancer. Molecules. 2014;19(9):15196–212.
Puma Biotechnology Inc. Nerlynx (neratinib): US prescribing information. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208051s000lbl.pdf. Accessed 19 July 2017.
Rabindran SK, Discafani CM, Rosfjord EC, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64(11):3958–65.
FDA. FDA approves neratinib for extended adjuvant treatment of early stage HER2-positive breast cancer [media release]. 17 Jul 2017. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm567259.htm.
Canonici A, Gijsen M, Mullooly M, et al. Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget. 2013;4(10):1592–605.
Menderes G, Bonazzoli E, Bellone S, et al. Efficacy of neratinib in the treatment of HER2/neu-amplified epithelial ovarian carcinoma in vitro and in vivo. Med Oncol. 2017;34(5):91.
Schwab CL, English DP, Black J, et al. Neratinib shows efficacy in the treatment of HER2 amplified carcinosarcoma in vitro and in vivo. Gynecol Oncol. 2015;139(1):112–7.
Lopez S, Cocco E, Black J, et al. Dual HER2/PIK3CA targeting overcomes single-agent acquired resistance in HER2-amplified uterine serous carcinoma cell lines in vitro and in vivo. Mol Cancer Ther. 2015;14(11):2519–26.
Shimamura T, Ji H, Minami Y, et al. Non-small-cell lung cancer and Ba/F3 transformed cells harboring the ERBB2 G776insV_G/C mutation are sensitive to the dual-specific epidermal growth factor receptor and ERBB2 inhibitor HKI-272. Cancer Res. 2006;66(13):6487–91.
Schwartz L, Croessmann S, Avogadri-Connors F, et al. Neratinib/fulvestrant but not fulvestrant alone maintain complete responses after treatment with trastuzumab/paclitaxel of mice bearing ER+/HER2+ xenografts [abstract no. 4818]. Cancer Res. 2017;77(13 Suppl.).
Montemurro F, Di Cosimo S, Arpino G. Human epidermal growth factor receptor 2 (HER2)-positive and hormone receptor-positive breast cancer: new insights into molecular interactions and clinical implications. Ann Oncol. 2013;24(11):2715–24.
Zhao XQ, Xie JD, Chen XG, et al. Neratinib reverses ATP-binding cassette B1-mediated chemotherapeutic drug resistance in vitro, in vivo, and ex vivo. Mol Pharmacol. 2012;82(1):47–58.
Breslin S, Lowry MC, O’Driscoll L. Neratinib resistance and cross-resistance to other HER2-targeted drugs due to increased activity of metabolism enzyme cytochrome P4503A4. Br J Cancer. 2017;116(5):620–5.
Hug B, Abbas R, Leister C, et al. A single-dose, crossover, placebo- and moxifloxacin-controlled study to assess the effects of neratinib (HKI-272) on cardiac repolarization in healthy adult subjects. Clin Cancer Res. 2010;16(15):4016–23.
Wang J, Li-Chan XX, Atherton J, et al. Characterization of HKI-272 covalent binding to human serum albumin. Drug Metab Dispos. 2010;38(7):1083–93.
Abbas R, Hug BA, Leister C, et al. Pharmacokinetics of oral neratinib during co-administration of ketoconazole in healthy subjects. Br J Clin Pharmacol. 2011;71(4):522–7.
Keyvanjah K, DiPrimeo D, Li A, et al. Pharmacokinetics of neratinib during coadministration with lansoprazole in healthy subjects. Br J Clin Pharmacol. 2017;83(3):554–61.
Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367–77.
Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in early-stage HER2+ breast cancer: 3-year analysis from a phase 3 randomized, placebo-controlled, double-blind trial (ExteNET) [abstract no. S5-02]. Cancer Res. 2016;76(4 Suppl 1).
Park JW, Liu MC, Yee D, et al. Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375(1):11–22.
Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28(8):1301–7.
Martin M, Bonneterre J, Geyer CE Jr, et al. A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2+ advanced breast cancer. Eur J Cancer. 2013;49(18):3763–72.
Freedman RA, Gelman RS, Wefel JS, et al. Translational Breast Cancer Research Consortium (TBCRC) 022: a phase II trial of neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2016;34(9):945–52.
Awada A, Colomer R, Inoue K, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2016;2(12):1557–64.
Chow LW, Xu B, Gupta S, et al. Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer. Br J Cancer. 2013;108(10):1985–93.
Saura C, Garcia-Saenz JA, Xu B, et al. Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2014;32(32):3626–33.
Awada A, Dirix L, Manso Sanchez L, et al. Safety and efficacy of neratinib (HKI-272) plus vinorelbine in the treatment of patients with ErbB2-positive metastatic breast cancer pretreated with anti-HER2 therapy. Ann Oncol. 2013;24(1):109–16.
Gajria D, Modi S, Saura C, et al. A phase I/II study of neratinib plus temsirolimus in HER2+ metastatic breast cancer reveals ongoing HER2 pathway dependence in many patients despite several lines of HER2 targeted therapy [abstract no. P5-19-04]. Cancer Res. 2015;75(9 Suppl.).
Freedman RA, Gelman RS, Melisko ME, et al. TBCRC 022: phase II trial of neratinib + capecitabine for patients (pts) with human epidermal growth factor receptor 2 (HER2+) breast cancer brain metastases (BCBM) [abstract]. In: ASCO Annual Meeting; 2017.
Abraham J, Puhalla SL, Sikov W, et al. NSABP FB-10: phase Ib dose-escalation trial evaluating trastuzumab emtansine (T-DMI) with neratinib (N) in women with metastatic HER2+ breast cancer (MBC) [abstract no. CT013]. Cancer Res. 2017;77(13 Suppl).
Jankowitz RC, Abraham J, Tan AR, et al. Safety and efficacy of neratinib in combination with weekly paclitaxel and trastuzumab in women with metastatic HER2positive breast cancer: an NSABP Foundation Research Program phase I study. Cancer Chemother Pharmacol. 2013;72(6):1205–12.
Ma CX, Bose R, Gao F, et al. Neratinib efficacy and circulating tumor DNA detection of HER2 mutations in HER2 non-amplified metastatic breast cancer. Clin Cancer Res. 2017. doi:10.1158/1078-0432.CCR-17-0900.
Hyman D, Piha-Paul S, Saura C, et al. Neratinib + fulvestrant in ERBB2-mutant, HER2-non-amplified, estrogen receptor (Ex positive, metastatic breast cancer (MBC): preliminary analysis from the phase II SUMMIT trial [abstract no. PD2-08]. Cancer Res. 2017;77(4 Suppl).
Sequist LV, Besse B, Lynch TJ, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(18):3076–83.
Gandhi L, Besse B, Mazieres J, et al. Neratinib ± temsirolimus in HER2-mutant lung cancers: an international, randomized phase II study [abstract no. MA04.02]. J Thorac Oncol. 2017;12(1 Suppl.):S358–9.
Jacobs SA, Lee JJ, George Jr TJ, et al. NSABP FC-7: a phase Ib study evaluating neratinib (N) and cetuximab (Cmab) in patients (pts) with quadruple wild-type (quad wt) (KRAS/NRAS/BRAF/PI3KCA wt) metastatic colorectal cancer (mCRC) resistant to Cmab [abstract no. C46]. Mol Cancer Ther. 2015;14(12 Suppl 2).
Wong KK, Fracasso PM, Bukowski RM, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15(7):2552–8.
Ito Y, Suenaga M, Hatake K, et al. Safety, efficacy and pharmacokinetics of neratinib (HKI-272) in Japanese patients with advanced solid tumors: a phase 1 dose-escalation study. Jpn J Clin Oncol. 2012;42(4):278–86.
Gandhi L, Bahleda R, Tolaney SM, et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J Clin Oncol. 2014;32(2):68–75.
Hyman D, Piha-Paul S, Rodon J, et al. Neratinib in HER2 or HER3 mutant solid tumors: SUMMIT, a global, multi-histology, open-label, phase 2 “basket” study [abstract no. CT001 and slide presentation]. Cancer Res. 2017;77(13 Suppl).
Ibrahim E, Tripathy D, Wilkinson M, et al. Effects of adding budesonide or colestipol to loperamide prophylaxis on neratinib-associated diarrhea in patients (pts) with HER2+ early-stage breast cancer (eBC): the CONTROL trial [abstract no. CT128 plus Poster]. Cancer Res. 2017;77(13 Suppl.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Emma Deeks is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information about this Adis Drug Review can be found at http://www.medengine.com/Redeem/00FBF0601C4AC36A.
Additional information
The original version of this article was revised: Under Section 3 the date 1 July 2017 was incorrect. This error has been corrected to 17 July 2017.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Deeks, E.D. Neratinib: First Global Approval. Drugs 77, 1695–1704 (2017). https://doi.org/10.1007/s40265-017-0811-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0811-4