Abstract
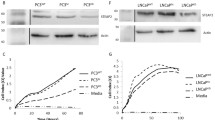
Transforming growth factor-β (TGFβ) is a secreted cytokine implicated as a factor in cancer cell migration and invasion. Previous studies have indicated that TGFβ isoforms may exert differential effects on cancer cells during different stages of the disease, however very little is known about the expression patterns and activity of the three isoforms in prostate cancer. Non-traditional signaling pathways including the PI3-Kinase have been associated with TGFβ-mediated effects on cancer cell invasion. In the present study, we have carried out expression analysis of TGFβ isoforms and signaling components in cell line models representing different stages of prostate cancer and studied the differential effects of specific isoforms on migratory and invasive behavior and induction of the PI3-kinase pathway. TGFβ1 and TGFβ3 were expressed in all cell lines, with TGFβ3 expression increasing in metastatic cell lines. Both TGFβ1 and TGFβ3 induced motility and invasive behavior in PC3 cells, however, TGFβ3 was significantly more potent than TGFβ1. TGFβRI and Smad3 inhibitors blocked TGFβ1 and TGFβ3 induced motility and invasion. TGFβ3 caused a significant increase in pAKTser473 in PC3 cells and PI3-kinase inhibitor LY294002 blocked TGFβ3 induced migration, invasion and phosphorylation of AKT. Both TGFβRI and Smad3 inhibitors blocked TGFβ3 induced pAKTser473. Based on these results, we conclude that TGFβ3 is expressed in metastatic prostate cancer cell lines and is involved in induction of invasive behavior in these cells. Furthermore, these effects of TGFβ3 are TGFβRI and Smad3 dependent and mediated via the PI3-kinase pathway.





Similar content being viewed by others
References
Wakefield LM, Roberts AB (2002) TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev 12(1):22–29
Padua D, Massague J (2009) Roles of TGFbeta in metastasis. Cell Res 19(1):89–102
Massague J, Blain SW, Lo RS (2000) TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103(2):295–309
Verrecchia F, Mauviel A (2002) Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol 118(2):211–215
Seoane J (2006) Escaping from the TGFbeta anti-proliferative control. Carcinogenesis 27(11):2148–2156
Derynck R, Akhurst RJ, Balmain A (2001) TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 29(2):117–129
Bierie B, Moses HL (2009) Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev 21(1):49–59
Joshi A, Cao D TGF-beta signaling, tumor microenvironment and tumor progression: the butterfly effect. Front Biosci 15: 180-94
Baardsnes J et al (2009) TbetaR-II discriminates the high- and low-affinity TGF-beta isoforms via two hydrogen-bonded ion pairs. Biochemistry 48(10):2146–2155
Piek E, Heldin CH, Ten Dijke P (1999) Specificity, diversity, and regulation in TGF-beta superfamily signaling. Faseb J 13(15):2105–2124
Rahimi RA, Leof EB (2007) TGF-beta signaling: a tale of two responses. J Cell Biochem 102(3):593–608
Laverty HG et al (2009) TGF-beta3 and cancer: a review. Cytokine Growth Factor Rev 20(4):305–317
Dickson MC et al (1995) Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development 121(6):1845–1854
Sanford LP et al (1997) TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 124(13):2659–2670
Proetzel G et al (1995) Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet 11(4):409–414
Kaartinen V et al (1995) Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet 11(4):415–421
Wrana JL et al (1992) TGF beta signals through a heteromeric protein kinase receptor complex. Cell 71(6):1003–1014
Cheifetz S et al (1990) Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J Biol Chem 265(33):20533–20538
De Crescenzo G et al (2003) Real-time monitoring of the interactions of two-stranded de novo designed coiled-coils: effect of chain length on the kinetic and thermodynamic constants of binding. Biochemistry 42(6):1754–1763
De Crescenzo G et al (2004) Enhancement of the antagonistic potency of transforming growth factor-beta receptor extracellular domains by coiled coil-induced homo- and heterodimerization. J Biol Chem 279(25):26013–26018
Lopez-Casillas F, Wrana JL, Massague J (1993) Betaglycan presents ligand to the TGF beta signaling receptor. Cell 73(7):1435–1444
Vilchis-Landeros MM et al (2001) Recombinant soluble betaglycan is a potent and isoform-selective transforming growth factor-beta neutralizing agent. Biochem J 355(Pt 1):215–222
Hart PJ et al (2002) Crystal structure of the human TbetaR2 ectodomain–TGF-beta3 complex. Nat Struct Biol 9(3):203–208
Van Themsche C et al (2007) Transforming growth factor-beta3 increases the invasiveness of endometrial carcinoma cells through phosphatidylinositol 3-kinase-dependent up-regulation of X-linked inhibitor of apoptosis and protein kinase c-dependent induction of matrix metalloproteinase-9. J Biol Chem 282(7):4794–4802
Karan D et al (2002) Expression profile of differentially-regulated genes during progression of androgen-independent growth in human prostate cancer cells. Carcinogenesis 23(6):967–975
Assinder SJ et al (2009) The TGF-beta, PI3 K/Akt and PTEN pathways: established and proposed biochemical integration in prostate cancer. Biochem J 417(2):411–421
Jiang BH, Liu LZ (2009) PI3 K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res 102:19–65
Salmena L, Carracedo A, Pandolfi PP (2008) Tenets of PTEN tumor suppression. Cell 133(3):403–414
Bakin AV et al (2000) Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 275(47):36803–36810
Lamouille S, Derynck R (2007) Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol 178(3):437–451
Shin I et al (2001) Transforming growth factor beta enhances epithelial cell survival via Akt-dependent regulation of FKHRL1. Mol Biol Cell 12(11):3328–3329
Vinals F, Pouyssegur J (2001) Transforming growth factor beta1 (TGF-beta1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-alpha signaling. Mol Cell Biol 21(21):7218–7230
Wilkes MC et al (2005) Transforming growth factor-beta activation of phosphatidylinositol 3-kinase is independent of Smad2 and Smad3 and regulates fibroblast responses via p21-activated kinase-2. Cancer Res 65(22):10431–10440
Zhong M et al (2010) Oxytocin induces the migration of prostate cancer cells: involvement of the Gi-coupled signaling pathway. Mol Cancer Res 8(8):1164–1172
Vo BT, Khan SA (2011) Expression of nodal and nodal receptors in prostate stem cells and prostate cancer cells: autocrine effects on cell proliferation and migration. Prostate 71(10):1084–1096
McDonald CA et al (2006) Follicle-stimulating hormone-induced aromatase in immature rat Sertoli cells requires an active phosphatidylinositol 3-kinase pathway and is inhibited via the mitogen-activated protein kinase signaling pathway. Mol Endocrinol 20(3):608–618
Massague J (1984) Type beta transforming growth factor from feline sarcoma virus-transformed rat cells. Isolation and biological properties. J Biol Chem 259(15):9756–9761
Reinhold D et al (1997) Inhibitors of dipeptidyl peptidase IV induce secretion of transforming growth factor-beta 1 in PWM-stimulated PBMC and T cells. Immunology 91(3):354–360
Jones E, Pu H, Kyprianou N (2009) Targeting TGF-beta in prostate cancer: therapeutic possibilities during tumor progression. Expert Opin Ther Targets 13(2):227–234
Amatschek S et al (2004) Tissue-wide expression profiling using cDNA subtraction and microarrays to identify tumor-specific genes. Cancer Res 64(3):844–856
Li C et al (1998) Role of transforming growth factor beta3 in lymphatic metastasis in breast cancer. Int J Cancer 79(5):455–459
Blanco-Aparicio C et al (2007) PTEN, more than the AKT pathway. Carcinogenesis 28(7):1379–1386
Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296(5573):1655–1657
Dubrovska A et al (2009) The role of PTEN/Akt/PI3 K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci USA 106(1):268–273
Cao C et al (2006) Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res 66(20):10040–10047
Festuccia C et al (2005) Molecular aspects of gefitinib antiproliferative and pro-apoptotic effects in PTEN-positive and PTEN-negative prostate cancer cell lines. Endocr Relat Cancer 12(4):983–998
Huang H et al (2001) PTEN induces chemosensitivity in PTEN-mutated prostate cancer cells by suppression of Bcl-2 expression. J Biol Chem 276(42):38830–38836
Acknowledgments
These studies were supported by the NIH/NCRR/RCMI grant #2G12RR003062, NIH P20 grant #5P20MD002285 and DOD Grant # W8I-08-1-0077.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walker, L., Millena, A.C., Strong, N. et al. Expression of TGFβ3 and its effects on migratory and invasive behavior of prostate cancer cells: involvement of PI3-kinase/AKT signaling pathway. Clin Exp Metastasis 30, 13–23 (2013). https://doi.org/10.1007/s10585-012-9494-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-012-9494-0