Abstract
Purpose
Addition of carboplatin (CBDCA) to neoadjuvant chemotherapy (NAC) in triple-negative breast cancer (TNBC) has improved pathological complete response (pCR) rates in previous studies. We present long-term survival outcomes (disease-free survival [DFS], pre-planned secondary endpoint; overall survival [OS], post hoc exploratory endpoint) of our randomized study of the addition of CBDCA to NAC for HER2-negative breast cancer.
Methods
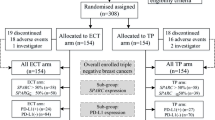
Patients with stage II/III, HER2-negative breast cancer (N = 179) were randomly assigned to receive CP–CEF (four 3-week cycles of CBDCA [area under the curve, 5 mg/mL/min, day 1] and weekly paclitaxel [wPTX, 80 mg/m2, day 1, 8, 15] followed by four 3-week cycles of cyclophosphamide, epirubicin, and 5-fluorouracil [CEF, 500/100/500 mg/m2]) or P–CEF (four cycles of wPTX followed by four cycles of CEF) as NAC. DFS and OS were analyzed at each population of pCR status and assigned treatment arm.
Results
Of 179 patients, 154 were available for long-term follow-up. At a median follow-up of 6.6 years (range, 0.7–8.0 years), patients who achieved pCR [n = 42, 23.5% (CP–CEF: n = 28, P–CEF: n = 16)] had longer DFS and OS than non-pCR patients [DFS; HR 0.15 (0.04–0.61), P = 0.008, OS; log-rank P = 0.003]. Addition of carboplatin to NAC significantly improved DFS and OS in the subset of patients with TNBC [DFS: HR, 0.22 (0.06–0.82), P = 0.015; OS: HR, 0.12 (0.01–0.96), P = 0.046], but not in the subset of patients with hormone receptor-positive disease or among all patients.
Conclusions
Addition of carboplatin to neoadjuvant chemotherapy significantly improved DFS and OS in patients with TNBC but not in those with hormone receptor-positive, HER2-negative breast cancer.



Similar content being viewed by others
References
von Minckwitz G, Schneeweiss A, Loibl S et al (2014) Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 15:747–756. https://doi.org/10.1016/S1470-2045(14)70160-3
Sikov WM, Berry DA, Perou CM et al (2015) Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 33:13–21. https://doi.org/10.1200/JCO.2014.57.0572
Loibl S, O’Shaughnessy J, Untch M et al (2018) Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol 19:497–509. https://doi.org/10.1016/S1470-2045(18)30111-6
Liedtke C, Mazouni C, Hess KR et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26:1275–1281. https://doi.org/10.1200/JCO.2007.14.4147
von Minckwitz G, Untch M, Blohmer J-U et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804. https://doi.org/10.1200/JCO.2011.38.8595
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (London, England) 384:164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Bonnefoi H, Litière S, Piccart M et al (2014) Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1–00 phase III trial. Ann Oncol 25:1128–1136. https://doi.org/10.1093/annonc/mdu118
Hahnen E, Lederer B, Hauke J et al (2017) Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol 3:1378–1385. https://doi.org/10.1001/jamaoncol.2017.1007
Loibl S, Weber KE, Timms KM et al (2018) Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response—final results from GeparSixto. Ann Oncol 29:2341–2347. https://doi.org/10.1093/annonc/mdy460
Sikov W, Berry D, Perou C et al (2016) Event-free and overall survival following neoadjuvant weekly paclitaxel and dose-dense AC +/− carboplatin and/or bevacizumab in triple-negative breast cancer: outcomes from CALGB 40603 (Alliance). Cancer Res 76:S2–05. https://doi.org/10.1158/1538-7445.SABCS15-S2-05
Ando M, Yamauchi H, Aogi K et al (2014) Randomized phase II study of weekly paclitaxel with and without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIA breast cancer without HER2 overexpression. Breast Cancer Res Treat 145:401–409. https://doi.org/10.1007/s10549-014-2947-1
Houssami N, MacAskill P, Von Minckwitz G et al (2012) Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer 48:3342–3354. https://doi.org/10.1016/j.ejca.2012.05.023
Symmans WF, Wei C, Gould R et al (2017) Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol 35:1010. https://doi.org/10.1200/JCO.2015.63.1010
Rugo HS, Olopade OI, DeMichele A et al (2016) Adaptive randomization of veliparib–carboplatin treatment in breast cancer. N Engl J Med 375:23–34. https://doi.org/10.1056/NEJMoa1513749
Telli ML, Timms KM, Reid J et al (2016) Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res 22:3764–3773. https://doi.org/10.1158/1078-0432.CCR-15-2477
Byrski T, Huzarski T, Dent R et al (2014) Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 147:401–405. https://doi.org/10.1007/s10549-014-3100-x
Lehmann BD, Jovanović B, Chen X et al (2016) Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS ONE 11:e0157368. https://doi.org/10.1371/journal.pone.0157368
Funding
This study was supported by the Health and Labour Sciences Research Grants (Clinical Cancer Research), Ministry of Health, Labour and Welfare (Grant Number: MHLW, 2009 Clinical Cancer Research General-020) and the Cancer Research and Development grants, and National Cancer Center (Grant Number: 2011-A-42).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MA has declared the conflicts related to personal fees from Astra Zeneca K. K., Novartis Pharma K. K., Chugai Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Eisai Co., Ltd., Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K. K., and Taiho pharmaceutical CO., LTD.; KA received personal fees as honoraria from Chugai Pharmaceutical, Eisai, AstraZeneca, Taiho Pharmaceutical, Novartis Pharma, Daiichi Sankyo, Mochida Pharmaceutical, Ono Pharmaceutical, and Eli Lilly Japan, and his institution received research funds from Chugai Pharmaceutical, Eisai and Sanofi.; TA reports personal fees from CHUGAI PHARMACEUTICAL CO., LTD and Pfizer Japan Inc during the conduct of the study.; KI reports personal fees from Eisai, Chugai, Pfizer, and Eli Lilly, and reports grants from Novartis, Pfizer, Chugai, Daiichi Sankyo, Parexel/Puma Biotechnology, MSD, Bayer, Eli Lilly, and Eisai.; AH reports personal fees from Chugai Pharmaceutical, AstraZeneca, Pfizer, Eli Lilly, Eisai, Daiichi Sankyo, and Novartis, outside the submitted work.; NM has received honoraria from Chugai, AstraZeneca, Pfizer, Eli Lilly, Eisai, and Takeda; funding from Chugai, AstraZeneca, Kyowa Hakko Kirin, MSD, Novartis, Pfizer, Eli Lilly, Eisai, and Daiichi Sankyo.; HY reports personal fees from AstraZeneca K.K., TAIHO PHARMACEUTICAL CO., LTD., Allergan Japan K.K., Pfizer Japan Inc., CHUGAI PHARMACEUTICAL CO., LTD., Novartis Pharma K.K., Shionogi & Co., Ltd., Takeda Pharmaceutical Co., Ltd., NIPPONKAYAKU CO., LTD., TOWA PHARMACEUTICAL CO., LTD., and Elsevier Japan KK during the conduct of the study.; TY reports personal fees as honoraria from Chugai, Eisai, Novartis, Taiho, Nippon Kayaku, AstraZeneca, Kyowa Kirin, Pfizer Japan, and Eli Lilly; and reports grants from Chugai, Taiho, Nippon Kayaku, and Kyowa kirin, outside the submitted work.; HI reports grants and personal fees from Chugai, personal fees from AstraZeneca, personal fees from Daiichi Sankyo, grants and personal fees from Novartis, grants from MSD, grants and personal fees from Lilly, personal fees from Kyowa Hakko Kirin, personal fees from Pfizer, during the conduct of the study; All other authors have declared no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2020_5580_MOESM1_ESM.png
Supplementary Fig.1: Comparison of the long-term survival of the intention-to-treat population according to pathological complete response (pCR) status.
10549_2020_5580_MOESM2_ESM.png
Supplementary Fig.2: Sensitivity analyses for hormone receptor-negative patients considering the use of additional adjuvant chemotherapy. Patients who received additional chemotherapy (n=19) were censored when the adjuvant treatment started.
10549_2020_5580_MOESM3_ESM.png
Supplementary Fig.3: The results of comparing prognosis between patients who completed and discontinued neoadjuvant chemotherapy. A Overall survival among all patients, B Disease-free survival among all patients, C Overall survival among HR-positive patients (n = 104), D Disease-free survival among HR-positive patients, E Overall survival among HR-negative patients (n = 75), F Disease-free survival in HR-negative patients.
Rights and permissions
About this article
Cite this article
Iwase, M., Ando, M., Aogi, K. et al. Long-term survival analysis of addition of carboplatin to neoadjuvant chemotherapy in HER2-negative breast cancer. Breast Cancer Res Treat 180, 687–694 (2020). https://doi.org/10.1007/s10549-020-05580-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05580-y