Abstract
Purpose
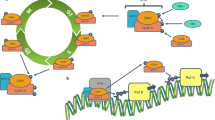
Deoxyribonucleic acid is wrapped around an octamer of core histone proteins to form a nucleosome, the basic structure of chromatin. Two main families of enzymes maintain the equilibrium of acetyl groups added to or removed from lysine residues. Histone deacetylases (HDACs) catalyze the removal of acetyl groups from lysine residues in histone amino termini and non-histone proteins also, leading to chromatin condensation and transcriptional repression. HDAC overexpression, resulting in tumor suppressor genes silencing, has been found in several human cancer tissues, indicating that aberrant epigenetic activity is associated with cancer development. Therefore, inhibitors of these enzymes are emerging anticancer agents and there is evidence supporting their role in hematological malignancies. The minimal efficacy of conventional chemotherapy has prompted a renewed focus on targeted therapy based on pathways altered during the pathogenesis of lung cancer. We identify the pleiotropic antitumor effects of HDAC inhibitors in lung cancer, focusing on the result caused by their use individually, as well as in combination with other chemotherapeutic agents, in lung cancer cell lines and in clinical trials.
Method
We searched reviews and original papers in Pubmed over the last 10 years.
Results
We identified 76 original papers on this topic.
Conclusions
Numerous preclinical studies have shown that HDAC inhibitors exhibit impressive antitumor activity in lung cancer cell lines. Nevertheless, Phase III randomized studies do not support HDAC inhibitors use in lung cancer patients in everyday practice. Ongoing and future studies would help determine their role in lung cancer treatment.

Similar content being viewed by others
Abbreviations
- HAT:
-
Histone acetyltransferase
- HDAC:
-
Histone deacetylase
- HDACIs:
-
Histone deacetylase inhibitors
- TKIs:
-
Tyrosine kinase inhibitors
- NF-kB:
-
Nuclear factor kB
- COX:
-
Cyclooxygenase
- HIF-1α:
-
Hypoxia-inducible factor-1-alpha
- VEGF:
-
Vascular endothelial growth factor
- EGFR:
-
Endothelial growth factor receptor
- TRAIL:
-
Tumor necrosis factor-related apoptosis inducing ligand
- IGFBP:
-
Insulin-like growth factor binding protein
- VPA:
-
Valproic acid
- NaB:
-
Sodium butyrate
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics 2012. CA Cancer J Clin 62:10–29. doi:10.3322/caac.20138
van Zandwijk N (2009) Lung cancer: oncogenesis and prevention. In: Spiro (ed) Thoracic malignancies, chapter 1. European Respiratory Society. Monograph 44:1–14. doi:10.1183/1025448x.00044001
Lawson MH, Eisen T (2009) The biology of lung cancer, thoracic malignancies, chapter 6. European Respiratory Society. Monograph 44:88–105. doi:10.1183/1025448x.00044006
Kopelovich L, Crowell JA, Fay JR (2003) The epigenome as a target for cancer chemoprevention. J Natl Cancer Inst 95(23):1747–1757
Bolden JE, Peart MJ, Johnstone RW (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5(9):769–784. doi:10.1038/nrd2133
Nakagawa M, Oda Y, Eguchi T, Aishima S, Yao T, Hosoi F et al (2007) Expression profile of class I histone deacetylases in human cancer tissues. Oncol Rep 18(4):769–774
Di Marcotullio L, Canettieri G, Infante P, Greco A, Gulino A (2011) Protected from the inside: endogenous histone deacetylase inhibitors and the road to cancer. Biochim Biophys Acta 1815(2):241–252. doi:10.1016/j.bbcan.2011.01.002
Zhong S, Fields CR, Su N, Pan YX, Robertson KD (2007) Pharmacologic inhibition of epigenetic modifications, coupled with gene expression profiling, reveals novel targets of aberrant DNA methylation and histone deacetylation in lung cancer. Oncogene 26(18):2621–3410. doi:1038/sj.onc.1210041
Barlési F, Giaccone G, Gallegos-Ruiz MI, Loundou A, Span SW, Lefesvre P et al (2007) Global histone modifications predict prognosis of resected non-small-cell lung cancer. J Clin Oncol 25(28):4358–4364. doi:10.1200/JCO.2007.11.2599
Miyanaga A, Gemma A, Noro R, Kataoka K, Matsuda K, Nara M et al (2008) Antitumor activity of histone deacetylase inhibitors in non-small cell lung cancer cells: development of a molecular predictive model. Mol Cancer Ther 7:1923–1930. doi:10.1158/1535-7163.MCT-07-2140
Zhu WG, Lakshmanan RR, Beal MD, Otterson GA (2001) DNA methyltransferase inhibition enhances apoptosis induced by histone deacetylase inhibitors. Cancer Res 61(4):1327–1333
Kim IA, Kim IH, Kim HJ, Chie EK, Kim JS (2010) HDAC inhibitor-mediated radiosensitization in human carcinoma cells: a general phenomenon? Radiat Res 51:257–263
Ellis L, Hammers H, Pili R (2009) Targeting tumor angiogenesis with histone deacetylase inhibitors. Cancer Lett 280(2):145–153. doi:10.1016/j.canlet.2008.11.012
Imre G, Gekeler V, Leja A, Beckers T, Boehm M (2006) Histone deacetylase inhibitors suppress the inducibility of nuclear factor-κΒ by tumor necrosis factor-α receptor-1 down-regulation. Cancer Res 66:5409–5418. doi:10.1158/0008-5472.CAN-05-4225
Adcock IM (2007) HDAC as anti-inflammatory agents. Br J Pharmacol 150(7):829–831. doi:10.1038/sj.bjp.0707166
Jeon HS, Lee SY, Lee EJ, Yun SC, Cha EJ, Choi E et al (2012) Combining microRNA-449a/b with a HDAC inhibitor has a synergistic effect on growth arrest in lung cancer. Lung Cancer 76(2):171–176. doi:10.1016/j.lungcan.2011.10.012
Oh SH, Whang YM, Min HY, Han SH, Kang JH, Song KH et al (2012) Histone deacetylase inhibitors enhance the apoptotic activity of insulin-like growth factor binding protein-3 by blocking PKC-induced IGFBP-3 degradation. Int J Cancer 131:2253–2263. doi:10.1002/ijc.27509
Ziauddin MF, Yeow WS, Maxhimer JB, Baras A, Chua A, Reddy RM et al (2006) Valproic acid, an antiepileptic drug with histone deacetylase inhibitory activity, potentiates the cytotoxic effect of Apo2L/TRAIL on cultured thoracic cancer cells through mitochondria-dependent caspase activation. Neoplasia 8(6):446–457. doi:10.1593/neo.05823
Perrino E, Cappelletti G, Tazzari V, Giavini E, Del Soldato P, Sparatore A (2008) New sulfurated derivatives of valproic acid with enhanced histone deacetylase inhibitory activity. Bioorg Med Chem Lett 18(6):1893–1897. doi:10.1016/j.bmcl.2008.02.007
Noro R, Miyanaga A, Shimokawa T, Kuribayashi H, Mizutani H, Minegishi Y et al (2009) The anticancer effect of histone deacetylase inhibitors and combination with the cytotoxic agents in lung cancer cells: biological analyses for future clinical application. J Nippon Med Sch 76(1):44–46
Amoêdo ND, Rodrigues MF, Pezzuto P, Galina A, da Costa RM, de Almeida FC et al (2011) Energy metabolism in H460 lung cancer cells: effects of histone deacetylas inhibitors. PLoS ONE 6(7):e22264. doi:10.1371/journal.pone.0022264
Kurtze I, Sonnemann J, Beck JF (2011) KRAS-mutated non-small cell lung cancer cells are responsive to either co-treatment with erlotinib or gefitinib and histone deacetylase inhibitors or single treatment with lapatinib. Oncol Rep 25(4):1021–1029. doi:10.3892/or.2011.1160
Kim HR, Kim EJ, Yang SH, Jeong ET, Park C, Lee JH, Youn MJ, So HS, Park R (2006) Trichostatin A induces apoptosis in lung cancer cells via simultaneous activation of the death receptor-mediated and mitochondrial pathway. Exp Mol Med 38(6):616–624
Platta CS, Greenblatt DY, Kunnimalaiyaan M, Chen H (2007) The HDAC inhibitor trichostatin A inhibits growth of small cell lung cancer cells. J Surg Res 142(2):219–226. doi:10.1016/j.jss.2006.12.555
Dombrowsky H, Barrenschee M, Kunze M, Uhlig S (2009) Conserved responses to trichostatin A in rodent lungs exposed to endotoxin or stretch. Pulm Pharmacol Ther 22(6):593–602. doi:10.1016/j.pupt.2009.08.005
Zhang F, Zhang T, Teng ZH, Zhang R, Wang JB, Mei QB (2009) Sensitization to γ-irradiation-induced cell cycle arrest and apoptosis by the histone deacetylase inhibitor trichostatin A in non-small cell lung cancer (NSCLC) cells. Cancer Biol Ther 8(9):823–831
Seo SK, Jin HO, Woo SH, Kim YS, An S, Lee JH, Hong SI, Lee KH, Choe TB, Park IC (2011) Histone deacetylase inhibitors sensitize human non-small cell lung cancer cells to ionizing radiation through acetyl p53-mediated c-myc down-regulation. J Thorac Oncol 6(8):1313–1319. doi:10.1097/JTO.0b013e318220caff
Wang X, Li G, Wang A, Zhang Z, Merchan JR, Halmos B (2011) Combined histone deacetylase and cyclooxygenase inhibition achieves enhanced antiangiogenic effects in lung cancer cells. Mol Carcinog 52(3):218–228. doi:10.1002/mc.21846
Sakuma Y, Yamazaki Y, Nakamura Y, Yoshihara M, Matsukuma S, Nakayama H, Yokose T, Kameda Y, Koizume S, Miyagi Y (2012) WZ4002, a third-generation EGFR inhibitor, can overcome anoikis resistance in EGFR-mutant lung adenocarcinomas more efficiently than Src inhibitors. Lab Invest 92(3):371–383. doi:10.1038/labinvest.2011.187
Hajji N, Wallenborg K, Vlachos P, Nyman U, Hermanson O, Joseph B (2008) Combinatorial action of the HDAC inhibitor trichostatin A and etoposide -mediated AIF-dependent apoptotic cell death in non-small cell lung carcinoma cells. Oncogene 27(22):3134–3144. doi:10.1038/sj.onc.1210976
Moody TW, Nakagawa T, Kang Y, Jakowlew S, Chan D, Jensen RT (2006) Bombesin/gastrin-releasing peptide receptor antagonists increase the ability of histone deacetylase inhibitors to reduce lung cancer proliferation. J Mol Neurosci 28(3):231–238. doi:10.1385/JMN:28:3:231
Shiau RJ, Chen KY, Wen YD, Chuang CH, Yeh SL (2010) Genistein and b-carotene enhance the growth-inhibitory effect of trichostatin A in A549 cells. Eur J Nutr 49(1):19–25. doi:10.1007/s00394-009-0044-8
Xu Q, Lu R, Zhu ZF, Lv JQ, Wang LJ, Zhang W, Hu JW, Meng J, Lin G, Yao Z (2011) Effects of tyroservatide on histone acetylation in lung carcinoma cells. Int J Cancer 128(2):460–472. doi:10.1002/ijc.25346
Crisanti MC, Wallace AF, Kapoor V, Vandermeers F, Dowling ML, Pereira LP, Coleman K, Campling BG, Fridlender ZG, Kao GD, Albelda SM (2009) The HDAC inhibitor panobinostat (LBH589) inhibits mesothelioma and lung cancer cells in vitro and in vivo with particular efficacy for small cell lung cancer. Mol Cancer Ther 8(8):2221–2231. doi:10.1158/1535-7163.MCT-09-0138
Edwards A, Li J, Atadja P, Bhalla K, Haura EB (2007) Effect of the histone deacetylase inhibitor LBH589 against epidermal growth factor receptor-dependent human lung cancer cells. Mol Cancer Ther 6(9):2515–2524. doi:10.1158/1535-7163.MCT-06-0761
Zhang W, Peyton M, Xie Y, Soh J, Minna JD, Gazdar AF, Frenkel EP (2009) Histone deacetylase inhibitor romidepsin enhances anti-tumor effect of erlotinib in non-small cell lung cancer (NSCLC) cell lines. J Thorac Oncol 4(2):161–166. doi:10.1097/JTO.0b013e318194fae7
Yang Y, Zhao Y, Liao W, Yang J, Wu L, Zheng Z, Yu Y, Zhou W, Li L, Feng J, Wang H, Zhu WG (2009) Acetylation of Fox01 activates bim expression to induce apoptosis in response to histone deacetylase inhibitor depsipeptide treatment. Neoplasia 11(4):313–324
Vinodhkumar R, Song YS, Devaki T (2008) Romidepsin (depsipeptide) induced cell cycle arrest, apoptosis and histone hyperacetylation in lung carcinoma cells (A549) are associated with increase in p21 and hypophosphorylated retinoblastoma proteins expression. Biomed Pharmacother 62(2):85–93
Vinodhkumar R, Song YS, Ravikumar V, Ramakrishnan G, Devaki T (2007) Depsipeptide a histone deacetylase inhibitor down regulates levels of matrix metalloproteinases 2 and 9 mRNA and protein expressions in lung cancer cells (A549). Chem Biol Interact 165(3):220–229
Hassig CA, Symons KT, Guo X, Nguyen PM, Annable T, Wash PL (2008) KD5170, a novel mercaptoketone-based histone deacetylase inhibitor that exhibits broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther 7(5):1054–1065. doi:10.1158/1535-7163.MCT-07-2347
Yu XD, Wang SY, Chen GA, Hou CM, Zhao M, Hong JA, Nguyen DM, Schrump DS (2007) Apoptosis induced by depsipeptide FK228 coincides with inhibition of survival signaling in lung cancer cells. Cancer J 13(2):105–113. doi:10.1097/PPO.0b013e318046eedc
Chang J, Varghese DS, Gillam MC, Peyton M, Modi B, Schiltz RL, Girard L, Martinez ED (2012) Differential response of cancer cells to HDAC inhibitors trichostatin A and depsipeptide. Br J Cancer 106(1):116–125. doi:10.1038/bjc.2011.532
Luchenko VL, Salcido CD, Zhang Y, Agama K, Komlodi-Pasztor E, Murphy RF, Giaccone G, Pommier Y, Bates SE, Varticovski L (2011) Schedule-dependent synergy of histone deacetylase inhibitors with DNA damaging agents in small cell lung cancer. Cell Cycle 10(18):3119–3128
Li CT, Hsiao YM, Wu TC, Lin YW, Yeh KT, Ko JL (2011) Vorinostat, SAHA, represses telomerase activity via epigenetic regulation of telomerase reverse transcriptase in non-small cell lung cancers. J Cell Biochem 112(10):3044–3053
Han S, Fukazawa T, Yamatsuji T, Matsuoka J, Miyachi H, Maeda Y, Durbin M, Naomoto Y (2010) Anti-tumor effect in human lung cancer by a combination treatment of novel histone deacetylase inhibitors: SL142 or SL325 and retinoic acids. PLoS ONE 5(11):e13834. doi:10.1371/journal.pone.0013834
Karelia N, Desai D, Hengst JA, Amin S, Rudrabhatla SV, Yun J (2010) Selenium-containing analogs of SAHA induce cytotoxicity in lung cancer cells. Bioorg Med Chem Lett 20(22):6816–6819. doi:10.1016/j.bmcl.2010.08.113
Komatsu N, Kawamata N, Takeuchi S, Yin D, Chien W, Miller CW, Koeffler HP (2006) SAHA, a HDAC inhibitor, has profound anti-growth activity against non-small cell lung cancer cells. Oncol Re 15(1):187–191
Noro R, Miyanaga A, Minegishi Y, Okano T, Seike M, Soeno C (2010) Histone deacetylase inhibitor enhances sensitivity of non-small-cell lung cancer cells to 5-FU/S-1 via down-regulation of thymidylate synthase expression and up-regulation of p21waf/cip1 expression. Cancer Sci 101(6):1424–1430
Casto BC, Pereira MA (2011) Prevention of mouse lung tumors by combinations of chemopreventive agents using concurrent and sequential administration. Anticancer Res (10): 3279–3284
Chien CW, Yao JH, Chang SY, Lee PC, Lee TC (2011) Enhanced suppression of tumor growth by concomitant treatment of human lung cancer cells with suberoylanilide hydroxamic acid and arsenic trioxide. Toxicol Appl Pharmacol 57(1):59–66
Seo SK, Jin HO, Lee HC, Woo SH, Kim ES, Yoo DH, Lee SJ, An S, Rhee CH, Hong SI, Choe TB, Park IC (2008) Combined effects of sulindac and suberoylanilide hydroxamic acid on apoptosis induction in human lung cancer cells. Mol Pharmacol 73(3):1005–1012. doi:10.1124/mol.107.041293
Boivin AJ, Momparler LF, Hurtubise A, Momparler RL (2002) Antineoplastic action of 5-aza-20-deoxycytidine and phenylbutyrate on human lung carcinoma cells. Anticancer Drugs 13(8):869–874
Shieh JM, Wei TT, Tang YA, Huang SM, Wen WL, Chen MY, Cheng HC, Salunke SB, Chen CS, Lin P, Chen CT, Wang YC (2012) Mitochondrial apoptosis and FAK signaling disruption by a novel histone deacetylase inhibitor, HTPB, in antitumor and antimetastatic mouse models. PLoS ONE 7(1):e30240
Hwang JJ, Kim YS, Kim MJ, Jang S, Lee JH, Choi J, Ro S, Hyun YL, Lee JS, Kim CS (2009) A novel histone deacetylase inhibitor, CG0006, induces cell death through both extrinsic and intrinsic apoptotic pathways. Anticancer Drugs 20(9):815–821. doi:10.1097/CAD.0b013e3283300a0f
Fournel M, Bonfils C, Hou Y, Yan PT, Trachy-Bourget MC, Kalita A, Liu J, Lu AH, Zhou NZ, Robert MF (2008) MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther 7(4):759–768. doi:10.1158/1535-7163.MCT-07-2026
Gray J, Cubitt CL, Zhang S, Chiappori A (2012) Combination of HDAC and topoisomerase inhibitors in small cell lung cancer. Cancer Biol Ther 13(8):614–622. doi:10.4161/cbt.19848
Tang YA, Wen WL, Chang JW, Wei TT, Tan YH, Salunke S, Chen CT, Chen CS, Wang YC (2010) Novel histone deacetylase inhibitor exhibits antitumor activity via apoptosis induction, F-actin disruption and gene acetylation in lung cancer. PLoS ONE 5(9):e12417. doi:10.1371/journal.pone.0012417
Zhang Q, Feng W, Zhou H, Yan B (2009) Advances in preclinical small molecules for the treatment of NSCLC. Expert Opin Ther Pat 19(6):731–751. doi:10.1517/13543770902967674
Niesen MI, Blanck G (2009) Rescue of major histocompatibility—DR surface expression in retinoblastoma-defective, non-small cell lung carcinoma cells by the MS-275 histone deacetylase inhibitor. Biol Pharm Bull 32(3):480–482
Belinsky SA, Grimes MJ, Picchi MA, Mitchell HD, Stidley CA, Tesfaigzi Y (2011) Combination therapy with vidaza and entinostat suppresses tumor growth and reprograms the epigenome in an orthotopic lung cancer model. Cancer Res 71(2):454–462. doi:10.1158/0008-5472.CAN-10-3184
Cuneo KC, Fu A, Osusky K, Huamani J, Hallahan DE, Geng L (2007) Histone deacetylase inhibitor NVP-LAQ824 sensitizes human non small cell lung cancer to the cytotoxic effects of ionizing radiation. Anticancer Drugs 18(7):793–800
Beckers T, Burkhardt C, Wieland H, Gimmnich P, Ciossek T, Maier T, Sanders K (2007) Distinct pharmacological properties of second generation HDAC inhibitors with the benzamide or hydroxamate head group. Int J Cancer 121(5):1138–1148
Tarasenko N, Nudelman A, Tarasenko I, Entin-Meer M, Hass-Kogan D, Inbal A, Rephaeli A (2008) Histone deacetylase inhibitors: the anticancer, antimetastatic and antiangiogenic activities of AN-7 are superior to those of the clinically tested AN-9 (Pivanex). Clin Exp Metastasis 25(7):703–716. doi:10.1007/s10585-008-9179-x
Wagner JM, Hackanson B, Lübbert M, Jung M (2010) Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clin Epigenet 1:117–136. doi:10.1007/s13148-010-0012-4
Schneider BJ, Kalemkerian GP, Bradley D, Smith DC, Egorin MJ, Daignault S (2012) Phase I study of vorinostat (suberoylanilide hydroxamic acid, NSC 701852) in combination with docetaxel in patients with advanced and relapsed solid malignancies. Invest New Drug 30(1):249–257. doi:10.1007/s10637-010-9503-6
Owonikoko TK, Ramalingam SS, Kanterewicz B, Balius TE, Belani CP, Hershberger PA (2010) Vorinostat increases carboplatin and paclitaxel activity in non-small cell lung cancer cells. Int J Cancer 126(3):743–755. doi:10.1002/ijc.24759
Marks PA, Richon VM, Kelly WK, Chiao JH, Miller T (2004) Histone deacetylase inhibitors: development as cancer therapy. Novartis Found Symp 259:269–281
Traynor AM, Dubey S, Eickhoff JC, Kolesar JM, Schell K, Huie MS, Groteluschen DL, Marcotte SM, Hallahan CM, Weeks HR, Wilding G, Espinoza-Delgado I, Schiller JH (2009) Vorinostat in patients with relapsed non-small cell lung cancer: a Wisconsin oncology network phase II study. J Thorac Oncol 4(4):522–526
Ramalingam SS, Maitland ML, Frankel P, Argiris AE, Koczywas M, Gitlitz B, Thomas S, Espinoza-Delgado I, Vokes EE, Gandara DR, Belani CP (2010) Carboplatin and Paclitaxel in combination with either Vorinostat or placebo for first-line therapy of advanced non-small cell lung cancer. J Clin Oncol 28(1):56–62. doi:10.1200/JCO.2009.24.9094
Mirshahidi HR, Hsueh CT (2010) Updates in non-small cell lung cancer-insights from the 2009 45th annual meeting of the American Society of Clinical Oncology. J Hematol Oncol 3:18. doi:10.1186/1756-8722-3-18
Vansteenkiste J, Van Cutsem E, Dumez H, Chen C, Ricker JL, Randolph SS, Schöffski P (2008) Early phase II trial of oral vorinostat in relapsed or refractory breast, colorectal, or non-small cell lung cancer. Invest New Drugs 26(5):483–488
Witta SE, Jotte RM, Konduri K, Neubauer MA, Spira AI, Ruxer RL, Varella-Garcia M, Bunn PA Jr, Hirsch FR (2012) Randomized phase II trial of erlotinib with and without entinostat in patients with advanced non-small-cell lung cancer who progressed on prior chemotherapy. J Clin Oncol 30(18):2248–2255. doi:10.1200/JCO.2011.38.9411
Juergens RA, Wrangle J, Vendetti FP, Murphy SC, Zhao M, Coleman B, Sebree R, Rodgers K, Hooker CM et al (2011) Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov 1(7):598–607. doi:10.1158/2159-8290.CD-11-0214
Candelaria M, Gallardo-Rincón D, Arce C, Cetina L, Aguilar-Ponce JL, Arrieta O, González-Fierro A et al (2007) A phase II study of epigenetic therapy with hydralazine and magnesium valproate to overcome chemotherapy resistance in refractory solid tumors. Ann Oncol 18:1529–1538
Scherpereel A, Berghmans T, Lafitte JJ, Colinet B, Richez M, Bonduelle Y, Meert AP, Dhalluin X, Leclercq et al (2011) European lung cancer working party (ELCWP). Valproate-doxorubicin: promising therapy for progressing mesothelioma. A phase II study, Eur Respir J 37(1): 129–135. doi:10.1183/09031936.00037310 10.1093/annonc/mdm204
Gridelli C, Rossi A, Maione P (2008) The potential role of histone deacetylase inhibitors in the treatment of non-small cell lung cancer. Crit Rev Oncol Hematol 68(1):29–36. doi:10.1016/j.critrevonc.2008.03.002
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petta, V., Gkiozos, I., Strimpakos, A. et al. Histones and lung cancer: are the histone deacetylases a promising therapeutic target?. Cancer Chemother Pharmacol 72, 935–952 (2013). https://doi.org/10.1007/s00280-013-2223-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2223-9