Abstract
OBJECTIVE To discuss the pathologic characteristics and the prognosis of neuroendocrine carcinoma of the bladder (NECB) in order to assist in making a precise diagnosis and to effectively treat the disease.
METHODS Clinicopathologic features of the 6 cases with NECB were analyzed retrospectively. The personal data of the patients and the pathologic and immunohistochemical characteristics of the tumor were investigated. The follow-up of these patients was conducted over a time period ranging from 3 months to 9 years.
RESULTS Transurethral resection of the bladder tumor was performed in all 6 patients. Based on the results of the pathologic examination, 4 of the cases were diagnosed as small cell neuroendocrine carcinoma and the other 2 were diagnosed as atypical carcinoid tumor. On immunohistochemical staining all tumors expressed neuroendocrine markers including neuronspecific enolase (CD56), synaptophysin (Syn) and chromogranin (CgA). In patients receiving partial cystectomy followed by postoperative chemotherapy, relapse was found in 4 of the 6 cases on follow up.
CONCLUSION NECB is a rare entity and a tumor with high malignant potential and characteristic pathologic features. Hematuria is the cardinal symptom of NECB. Metastasis may occur at an early stage and the prognosis of the disease is poor. Final diagnosis of NECB depends on histopathologic examination and immunohistochemical assays. Surgical excision combined with radiotherapy and chemotherapy is considered an effective treatment.
keywords
Introduction
Bladder neuroendocrine carcinoma (NECB) is a rare malignant bladder tumor. Invasion and metastasis occur frequently because the tumor is typically poorly differentiated and inherently highly malignant. During a period from 1999 to 2008, 6 patients with NECB were treated in our hospital. In our study, the clinicopathologic features of the disease were studied in combination with previous literature on NECB.
Materials and Methods
Materials
Data from the 6 cases of NECB during the 9-year period, i.e. from 1999 to 2008, were collected. Of the NECB patients, 5 were male and 1 was female, with the average age of 61 years; the age ranged from 50 to 72. In the 6 cases, painless hematuria was seen in 5 via macroscopic observation, and dysuria occurred in 1. Symptoms such as flushed complexion, arrhythmia, diarrhea and paroxysmal hypertension etc., did not occur in any of the 6 cases, and a history of the tumor in other organs was not found.
Diagnostic criteria
i) The tumor originates from the urinary bladder, and it is not found in the other endocrine organs or tissues; ii) The morphologic characteristics of neuroendocrine tumors are present; iii) One or more of the neuroendocrine tumor markers in immunohistochemical assays are positive, i.e. chromogranin A (CgA), synaptophysin (Syn) and CD56 nerve cell adhesion factor.
Methods and judgment of the result
The H&E sections were prepared as follows. A 4% neutral formalin fixation, paraffin imbedding and H&E staining were performed on the excised tumor specimen. Immunohistochemical S-P method was used to detect tumor markers, and the results showed positive expression of tumor markers in various degrees. Brown-yellow staining localized in the cytoplasm and cell membranes signified CgA, Syn and CD56 positivity; similar staining localized to the cell nucleus indicated CK positivity. Antibodies from Fuzhou Maixin Biological Technology Co., Ltd. were used.
Results
Clinical features
Among the 6 patients, hematuria was the most common clinical manifestations of the malignancy in occurrence. Based on the size of the tumor, partial or total cystectomy was performed, accompanied by intravesical chemoembolization.
Pathologic examination
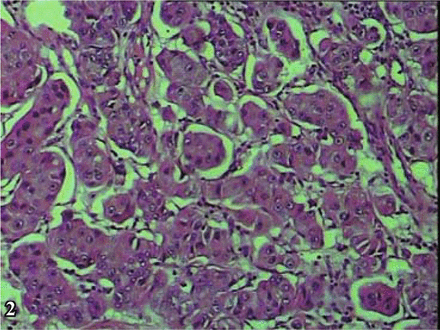
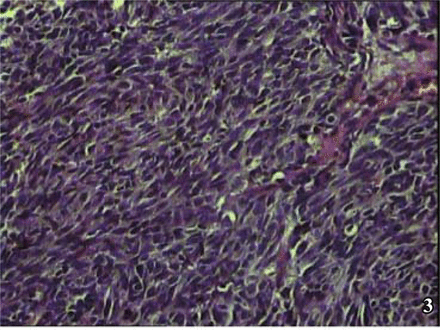
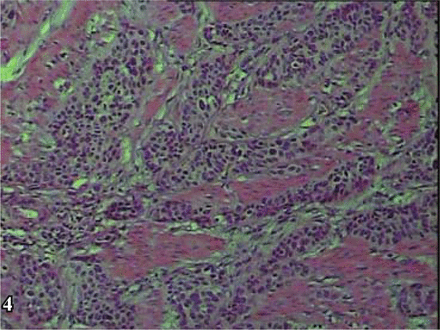
The results of macroscopic observation indicated that in 4 of the 6 NECB cases, the tumor presented as a polypoid intracavitary protrusion. The diameter of the tumor ranged from 1 cm-4 cm, and the cut surface was firm and grayish with a flaky texture with a resemblance to fish. The tumors were not well-circumscribed, and the margins with the surrounding tissue were indistinct. Central hemorrhagic necrosis was seen in some cases with invasion into the urinary bladder wall. Microscopic examination showed that the cancer cells formed a diffusely patchy or an irregular alveolar arrangement. The neoplasm also commonly presented in a trabecular band-like distribution or occasionally a pseudo-rosette-like array. The mesenchyma was highly vascular. The 4 cases with small cell carcinoma (SCC) (Fig. 1) were found to concurrently have transitional cell carcinoma (TCC) mixed with the SCC. Concomitant differentiation of adenocarcinoma and squamous carcinoma was shown in 1 of these 4 cases. In the 4 cases, 2 were atypical carcinoids, in which 1 was acidophil atypical carcinoid tumor (Fig. 2) and the other was fusiform-cell, atypical carcinoid tumor (Fig. 3). In the 6 NECB cases, invasion into the muscular layer of the bladder occurred in 2 (Fig. 4), and infiltration to the outer membrane of the bladder in the other 4. Further, in these 4 cases concurrent lymphatic metastasis was seen in 2 and intravascular cancer embolus in the other 2. Areas of typical carcinoid tumor were not found in any of the cases, which was in accordance with the reports in the literature.
Small cell carcinoma of the bladder: small cells with little cytoplasm, H&E × 200.
Atypical oncocytic carcinoid: alveolar arrangement of cells, eosinophilic granular cytoplasm and vascular stroma, H&E × 200.
Atypical spindle cell carcinoid: tumor cells are mainly spindle cells, with accordant size, H&E × 200.
Small cell carcinoma of the bladder: infiltration of small cell carcinoma into the muscular layer, H&E × 200.
Immunohistochemistry
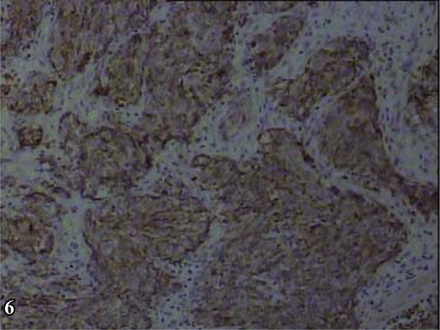
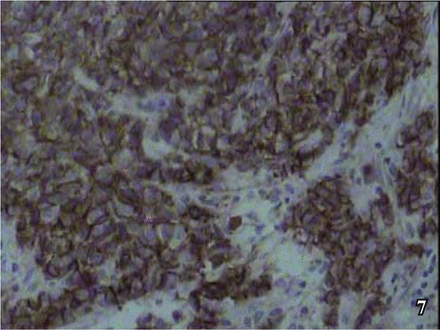
Positive expression at various degrees was shown by the neuroendocrine tumor markers, i.e., diffusely granular positive expression of Syn in the cytoplasm (Fig. 5), granular positive CgA in the cytoplasm (Fig. 6), diffusely positive CD56 in the cell membranes (Fig. 7), and positive CK in the cell nuclei (Fig. 8).
Immunohistochemical staining of synaptophysin indicated the tumor cytoplasm was positive, S-P × 200.
Immunohistochemical staining of chromogranin A showed the tumour cytoplasm was positive, S-P × 200.
Immunohistochemical staining of CD56 showed bladder neuroendocrine carcinoma, and cell membrane was positive, S-P×200.
Immunohistochemical staining of Cytokeratin showed bladder neuroendocrine carcinoma, and tumour cytoplasm was positive, S-P× 200.
Follow-up
The follow-up period of the 6 cases ranged from 3 months to 9 years. In 1 of the cases, the patient history was short, i.e., only 2 months, and failure to follow-up occurred in 2 cases. Recurrence was found in all 4 cases during the follow-up period. Of the 4 cases, relapse occurred once in 1, twice in 2 and four times in 1 patient. The average time of the relapse in the 6 patients was 18.3 months from the time of the first diagnosis. Of these patients, metastasis to the neighboring organs such as prostate or rectum occurred in 1, and death occurred in 1 owing to metastasis to distant gastrointestinal organs and to the lymph nodes of the neck.
Discussion
Neuroendocrine carcinoma is a rare malignancy in urinary bladder, which is most commonly seen in older males at greater than 60 years of age. The malignancy belongs to the APUD classification of tumors, and can occur in multiple organs and tissues of the body, with a predilection for the alimentary tract. Neuroendocrine carcinoma of the bladder (NECB) is rarely seen. Since NECB is classified as an endocrine tumor, it can secrete hormones such as 5-HT and histamine etc., and can result in the so-called carcinoid syndrome, with clinical symptoms such as diarrhea, flushing, asthma, cyanosis, etc. NECB can be classified into 3 types based on cellular ontogeny. In the majority of cases of NECB, argentaffin granules are present when the disease occurs in the foregut, while 5-HT is minimal and the tumor is not accompanied by carcinoid syndrome. Argentaffin granules are found when NECB occurs in the midgut, with profuse 5-HT, resulting in typical carcinoid syndrome. Granules are not found at onset of the disease occurring in the hindgut, and as a result, carcinoid syndrome is not a presenting feature in these cases. The urinary bladder evolves from the ventral part of cloacal chamber, and the cloacal chamber evolves from dilated part of the hindgut’s extremity. So NECB including carcinoid tumors, are traceable to the hindgut[1,2]. In our group, hematuria was the most common clinical symptom presenting with NECB, while carcinoid syndrome was not seen, and osseous metastasis occurred infrequently.
There are 4 generally accepted approaches regarding the histogenesis of NECB: i) cancerization of the neuroendocrine cells, as we know that neuroendocrine cells exist in transitional epithelium of the normal or metaplastic mucous membrane of urinary bladder (MMUB)[3]; ii) neural crest cells are under the MMUB and inside the muscular layer, so NECB can grow under the mucous membrane and within the muscular layer; iii) glandular epithelial metaplasia of MMUB; iv) primary multipotential stem cells in the basal layer of transitional epithelium[4] which can differentiate to SCC and non-small cell carcinoma. At present, most researchers support the idea; therefore, we can say that endocrine carcinoma of the bladder is often complicated by the components of malignant epithelial tumor[5]. In the 6 cases in our study, cystitis glandularis in the mucous membrane alongside the tumor was found in 1, complicated papillary transitional cell carcinoma in 3, and concurrent adenocarcinoma and differentiated squamous carcinoma in 1, with submucosal and intramuscular layer infiltration. Neuroendocrine carcinoma may occur together with carcinoma in situ, infiltrative TCC, adenocarcinoma and squamous carcinoma, which is in accordance with the reports in the literature[6-8].
The clinical manifestations of NECB are similar to those of the commonly seen malignant tumors of bladder, including painless hematuria in the majority of the cases, and dysuria in the minority. Papillary or polypoid progression of the tumor can often be seen by macroscopic observation, and tumors, including wide based type tumors, may occur in any site, with the diameter ranging from 0.5 to 5 cm. Multiple tumors can frequently be seen in NECB cases. Diagnosis of the tumors is mainly based on histologic examination and immunohistochemical assays, and there are various degrees of cellular differentiation of neuroendocrine carcinoma similar to pulmonary tumors of the same kind, i.e. the well-differentiated typical carcinoid, moderately differentiated atypical carcinoid, the poorly differentiated SCC, etc. In the 6 NECB cases in our study, morphologic characteristics of neuroendocrine tumors of various degrees were shown by light microscopy as follows: diffusely patchy or irregularly alveolar arrangement, and sometimes trabecular bandlike distribution, with obviously visible mitoses. In our group, 4 of the 6 cases were small cell neuroendocrine carcinoma, and the other 2 were atypical carcinoid, among which 1 was acidophil cell atypical carcinoid and 1 was fusiform cell atypical carcinoid. SCC has the characteristics of small cell, less cytoplasm and inconspicuous nucleolus. The acidophilic cell atypical carcinoid may have moderately eosinophilic granular cytoplasm with fine granular nuclear chromatin. Some of the cells of the fusiform cell atypical carcinoid were spindle-shaped and presented in a fascicular arrangement. SCC is more invasive than atypical carcinoid, and mitoses and necrosis frequently occur. In the follow-up of the 4 patients with SCC, both had multiple relapses and lymphatic metastasis could be seen with extensive tumor metastasis in the multiple neighboring organs in 1 of the 4 cases. No typical carcinoids were found among the cases of our group. Diagnosis of the disease can be made based on the immunohistochemical markers Syn, CgA and CD56, and the typical histologic characteristics, with one or more positive neuroendocrine markers and a positive epithelial marker CK.
Regarding the diagnosis of NECB, distinguishing from the following tumors is necessary. i) Poorly differentiated transitional cell carcinoma: there is a tendency of alveolar arrangement of cells, apparent pleomorphism of the cells, comparatively dark nuclear chromatin, negative reactivity with immunohistochemical neuroendocrine markers, and frequent positive expression of the CK7 in transitional cells. ii) Malignant lymphoma: sub-mucosal infiltration is the most typical clinical manifestation, and concurrent TCC is rarely seen. Positive LCA is the commonly used differential stain in immunohistochemistry; iii) Differentiation of NECB from metastatic SCC and neuroendocrine carcinoma, from other sites such as lung etc., is needed. Case history and clinical imaging should be checked to see if there is evidence a concurrent epithelial tumor. Moreover, more tissue should be taken from the specimens in order to rule out the possibility of other malignant tumors. iv) Differentiation with pheochromocytoma (paraganglioma) is also necessary. Pheochromocytoma is one of the neuroendocrine tumors, however, the characteristics of this tumor include a predilection for young women, and clinical manifestations such as hematuria, dizziness, headache, arrythmia, and hyperhidrosis are commonly seen. Further, a transient rise in blood pressure can occur after urination. Histologic examination has shown that the tumor consists of homologous tumor cells of which the principal cell is rounded or polygonal shaped and the cytoplasm of the cell contains abundant eosinophilic granules. Mitoses are rare, and there are very few fusiform supporting cells in the tumor. The tumor markers CgA and Syn, as well as the supporting cells S-100 are positive, and CK and EMA are negative, which are the major points of differentiation.
The pathologic manifestations of NECB are usually accompanied by atypical transitional epithelial hyperplasia, carcinoma in situ, TCC, squamous cell carcinoma and adenocarcinoma to form a heterogenous tumor, histologically. Since the quantity of tissue from pathology biopsies is generally limited, misdiagnosis of neuroendocrine carcinomas occurs from time to time, which reminds pathologists to make the diagnosis with caution.
In the treatment of NECB, surgery plus chemotherapy or regional radiotherapy is usually conducted. Surgery should be considered as the preferred treatment, and the extent of the surgery depends on the size and site of the tumor, the depth of invasion and the status of lymph node metastasis. Urocystic resection per urethra, partial resection, or total cystectomy with lymphadenectomy of the pelvic cavity can be performed[9,10], and systemic administration of chemotherapeutic agents should be prioritized in the treatment regimen. At present, the commonly used chemotherapeutics include cisplatin, adriamycin, cyclophosphamide and VP-16, but chemotherapeutic regimens vary greatly. Nevertheless, combined chemotherapy is superior to a single medication. In addition, reports from China recommend that[11] chemotherapeutic drugs can be administered via internal iliac-arterial duct after radical excision of the bladder, since this method may raise the drug concentration of local chemotherapy, with less side effects to the body thus increasing chemotherapeutic safety. Further, the combination of surgery, including radical excision, partial resection and excision of the tumor per urethra, chemotherapy (systemic combination chemotherapy or internal iliac arterial cannula chemotherapy), regional radiotherapy, and various therapeutic alliances, can prolong the survival time of patients with NECB.
Since the number of the cases in this study was small, there was only limited follow-up information in some of the cases; therefore, we failed to conduct a proper assessment of the clinical behavior and prognosis of NECB. It has been reported in the previous literature that long-term survival may be possible following simple surgery; however, infiltration and metastasis readily occur owing to high potential malignancy.
- Received May 20, 2009.
- Accepted July 21, 2009.
- Copyright © 2009 by Tianjin Medical University Cancer Institute & Hospital and Springer