keywords
Case Report
A 38-year-old woman underwent a hysterectomy for a uterine tumor. A giant circumscribed nodule with a diameter of 20 cm at its widest point was found within the myometrium of the uterine corpus without hemorrhage or necrosis. More than 10 specimens were pathologically examined. Eleven years after hysterectomy, she developed five tumors (the largest diameter = 2 cm) in the right lung and retroperitoneal tumor (the largest diameter = 9.5 cm). Microscopic examination indicated uterine, retroperitoneal and pulmonary foci, which were characterized by a benign appearance, including nodular smooth-muscle proliferation. The smooth muscle cells had an oval shape with relatively clear cytoplasm and a few irregular nuclei. In each specimen, spindle-shaped cells showed an interlacing bundle pattern with intermediated cellularity. The mitotic index was very low and no nuclear atypia was observed (Fig.1). Even though the serial pathological examination did not indicate any malignant potential, we diagnosed this case as benign metastasizing leiomyoma (BML).
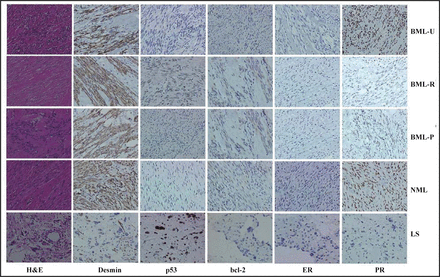
H&E staining and immunostaining of BML-U, BML-R, BML-P, NML, and LS. p53, ER and PR distributed to the nucleus, whereas desmin and bcl-2 were found in the cytoplasm.
In the present case, pulmonary (BML-P) and retroperitoneal (BML-R) metastases and primary foci (BML-U) of uterine leiomyoma were examined with one case of non-metastasizing leimyoma of the uterus (NML) and one case of leiomyosarcoma (LS) as control. All tissues were fixed in 4% neutralized formaldehyde, embedded in paraffin and cut into 4 μm sections. These sections were stained by H&E and then immuhistochemically stained using antibodies against desmin (DAKO, USA; 1:100), p53 (DAKO, USA; 1:100), bcl-2 (DAKO, USA;1:80), ER (NovoCastra, UK;1:80), and PR (Novo Castra, UK;1:100) antibodies. P53, ER and PR stained the nucleus, whereas desmin, bcl-2 stained the cytoplasm. Immunostaining showed that smooth muscle cells of the BML strongly expressed the desmin protein, in accordance with the NML (Fig.1). As shown in the Fig.1, primary or metastatic foci of the BML exhibited an intermediate expression pattern of p53, bcl-2, ER and PR between NML and LS. There was strong expression of p53 and bcl-2 in the retroperitoneal or pulmonary foci of the BML respectively, compared with the primary foci. The converse was true for the PR.
Discussion
Uterine leiomyoma, the commonest gynecological neoplasm in women of reproductive age, has a mortality of about 50% for women over 30. The majority of these tumors are benign with the relative frequency of leiomyosarcomas, estimated to be between 0.13% and 6%. Very unusual uterine leiomyomas develop as rare intravascular or peritoneal leiomyomatosis, which are called “benign metastasizing leiomyoma”[1]. Steiner et al.[2] for the first time reported that BML, which showed multiple, nodular smooth-muscle proliferation, frequently located in the lung, lymph node, or abdomen, and appeared to be derived from a histologically benign-looking leiomyoma of the uterus. Until now, the origin of this rare lesion continues to be somewhat controversial.
Horstmann et al.[3] initially proposed the term “benign metastasizing leiomyoma” and approximately 90% of the cases reported since then support a myometrial origin for these tumors. There were three areas of experimental evidence described as follows: Anatomically, the uterus showed widespread microvascular invasion by benign muscle-fibroid tissue in a patient with a uterine leiomyoma and thrombus, consisting of proliferating smooth muscle fibers found in the inferior vena cava[4]. Morphologically, uterine and pulmonary tumors have been found to exhibit similar features, such as multiple or single, solid, spatially well-delimited tumor masses, which are composed of spindle-shaped, rather densely packed cells[5]. Genetically, primary and metastatic foci express both estrogen and progesterone receptors with identical X-chromosome inactivation and a balanced karyotype [6,7]. These findings support the concept that BML is a clonal metastasizing process.
Another proposal also has been put forward that mulitifocal smooth-muscle proliferations might be the result of a systemic leiomyomatosis. As we know, the benign leiomyoma of the uterus was removed including a large amount of surrounding tissue, so it was very difficult for it to metastasize. On the other hand, leiomyomas develop simultaneously in the uterus, lung and elsewhere[8]. Generally, detection of the metastasis from leiomyoma takes considerable time after removal of the primary foci of the uterus. No sufficient evidence excluded the possibility of leiomyomatosis.
The last assumption was that BML might represent a heterogeneous group of smooth muscles tumors, ranging from leiomyoma to low-grade leiomyosarcoma, or should be classified as leiomyoma with low malignant potential[9]. It was reported that the S-phase fraction of BML was near the upper limit of the range in seven ordinary uterine leiomyomas, and was distinctly lower than that in a uterine leiomyosarcoma[9]. This kind of uterine leiomyoma also displays metastatic properties, which are typical characteristics of malignancies, like leiomyosarcoma. Therefore, it is also reasonable to consider BML as a special kind of leiomyosarcoma. In this study, we also found that primary, or metastatic foci of BML showed an intermediate expression pattern of p53, bcl-2, ER and PR between NML and LS, providing new evidence for the hypothesis described above.
Additionally, we found more intense expression of bcl-2 and p53 in pulmonary or its retroperitoneal metastases of BML, compared with primary foci. The heterozygous expression pattern gave us a new clue for the metastatic process. For the first possibility, microinvasion took place in the uterine leiomyoma with metastatic potential before being removed. When abnormal genetic alteration accumulated to some degree, the microinvasive leiomyoma would grow and metastasize outside the uterus. For the second possibility, the pulmonary and retroperitoneal leiomyomatosis had low potential of proliferation, compared with that of the uterus. Consequently, more genetic alteration resulted in large growth that could be clinically found. Taken together, aberrant genetic accumulation played an important role in the pathogenesis of BML. On the other hand, the BML-R and BML-P had different expression levels of p53 and ER, suggesting that expression of both proteins might be regulated by the microenvironment.
To our knowledge, the patient underwent a long period of follow-up until diagnosis for BML. If feasible, a radical parenchyma-sparing surgical resection is advocated as the treatment of first choice. It was documented that a menopausal or pregnant patient can experience spontaneous regression of a BML, which was correlated with the serum level of estrogen and progesterone[10]. These clinical phenomena suggested that BML was hormone dependent and hormonal therapy might be the best option for unresectable metastatic disease after surgically removing enough of the BML to influence the normal physiological processes. However, the use of hormone inhibitors on a patient with BML should be carefully considered because the curative effect of the therapy depends on the expression of hormone receptors. Therefore, it is necessary to examine the expression of ER and PR using biopsy, or surgical sampling before determination of the treatment method. In this study, we found different expression levels of ER and PR in metastases at different sites[10]. This finding suggests that hormone therapy possibly has different outcomes for different organs with BML.
- Received November 20, 2007.
- Accepted January 11, 2008.
- Copyright © 2008 by Chinese Anti–Cancer Association