Abstract
OBJECTIVE To study whether siRNA targeting against the Bcl-2 gene can enhance sensitivity of HL-60 cells to all trans retinoic acid (ATRA).
METHODS siRNA, which is a leading sequence selected by previous experiments, was transferred into HL-60 cells. At 6 h after transfection, the cells were cultured with ATRA. The cell growth of the HL-60 cells was measured by the MTT assay at 24, 48, 72 h. The level of the Bcl-2 protein and ROS (reactive oxygen species) as well as membrane potential of the mitochondria were determined by flowcytometry.
RESULTS siRNA significantly increased the inhibitory effect of ATRA on growth of the HL-60 cells. The combination of siRNA with ATRA resulted in a decrease in the Bcl-2 protein level and an increase in the ROS level as well as significantly lowering the mitochondrial membrane potential of the HL-60 cells (P < 0.05).
CONCLUSION Effective siRNA targeting of Bcl-2 increases the sensitivity of HL-60 leukemic cells to ATRA by inhibiting the expression of the Bcl-2 protein.
keywords
Introduction
Expression of the Bcl-2 protein has been observed in a majority of human cancer specimens and cell lines. A high level of expression of Bcl-2 is associated with resistance to chemotherapeutic agents in a number of tumor types, so that a drug which would reduce the levels of this protein might be expected to promote apoptosis and therefore be considered to be a promising chemotherapeutic agent[1-5]. The Bcl-2 antisense oligonucleotide, G3139 (Gensense, Oblimersen), which has been developed by Genta Inc, is currently in phase I~III clinical trials[4-7].
Double-stranded RNA-dependent post-transcriptional gene silencing, also known as RNA interference (RNAi), is a phenomenon where double-stranded small interfering RNA (siRNA) complexes can target specific genes. A 20-nt siRNA is long enough to produce RNAi[8-10]. Recently some experiments in vivo and in vitro have verified that siRNA can induce post-transcriptional homologous gene silencing in mammalian cells[8-12].
In our lab, by using T7 in vitro transcription we have identified 4 siRNAs that target against Bcl-2 effectively and down-regulate the Bcl-2 protein[13-15]. In this study, we further investigated whether Bcl-2 siRNA could increase the sensitivity to all trans retinoic acid (ATRA) in HL-60 cells, which might be helpful for searching for a new drug against the Bcl-2 protein.
Materials and Methods
Reagents
Antibodies against the Bcl-2 protein were purchased from Santa Cruz. RPMI1640 and newborn calf serum were obtained from Gibco BRL. In our lab, four effective siRNA sequences targeted against Bcl-2 mRNA have been identified by in vitro transcription synthesis. The most effective siRNA was used for the present experiment. G3139 oligos and siRNA of G3139 (GsiRNA) sequences targeted against Bcl-2 mRNA were also used for comparison.
Cell culture and transfection with the siRNA
The HL-60 cell line was purchased from the Shanghai Cell Bank. The cells were cultured in RPMI medium supplemented with 10% heat-inactivated fetal calf serum at 37°C under 5% CO2 in a humidified incubator. Lipofectamine 2000 was used for siRNA transfections. HL-60 cells in the exponential phase of growth were grown for 6 h, then transfected with siRNA. The cells were plated in antibiotic-free OPTI-MEM at a density of 1 × 104 cells/mL. The HL-60 cells were transfected with either Bcl-2siRNA (0.1 μmol/L), or GsiRNA (0.1 μmol/L). In addition to medium controls and vehicle control (Lipofectamine 2000), the cells were incubated with ATRA (1 μmol/L) or ATRA (1 μmol/L) + siRNA (0.1 μmol/L). At 24, 48, 72 and 96 h after transfection, silencing was examined.
Quantificaion of the Bcl-2 protein by flow cytometry
Treated HL-60 cells and untreated HL-60 cells were collected by centrifugation (2,000 ×g) for 5 min. The cells were washed twice and fixed in 4% formaldehyde (Sigma) for 30 min on ice, followed by permeabilization with 0.1% Triton X in a specific PBS (s-PBS, containing 1% human AB serum, 1% Tween-20, pH 7.2) 10 min at 4°C. Cells were then washed in cold phosphate-buffered saline (PBS) before being added to 10 μl of anti-Bcl-2 antibody (1:100) (DAKO, Carpinteria, CA) for 30 min at room temperature in the dark. After washing twice with PBS, 10 μl of fluorescent isothiocyanate (FITC)-conjugated second antibody (1:100) was added. After washing twice again with PBS, the cells were examined by flow cytometry (Becton Dickinson)and the percentage of cells with positive staining for the Bcl-2 protein was determined.
Assay of cell viability
The effects of ATRA on HL-60 cells were estimated after Bcl-2 siRNA transfection to determine whether the Bcl-2 siRNAs enhanced sensitivity of the HL-60 cells to ATRA. The experiment was divided into six groups: control, G3139 (15 μmol/L), GsiRNA, siRNA, ATRA and ATRA + siRNA. After treatment, the cells were incubated at 37°C. At 24, 48, 72, 96 h, MTT solution was added (5 mg/ml) followed by an incubation of 4 h, after which, the samples were centrifuged at 2000 rpm for 5 min. After removing the supernatant, 200 μl of DMSO was added to dissolve the crystals followed by measurement of the absorbance at 490 nm.
Detection of the intrinsic ROS levels and mitochondrial membrane potential
The HL-60 cells were harvested at 48 h after treatment with Bcl-2 siRNAs, followed by addition of ATRA. 2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma, USA) was used for ROS capture. The change in the mitochondrial membrane potential (ΔΨm) was examined by staining the cells with a mitochondrial specific, fluorescent dye, Rh123. After incubation with DCFH-DA and RH123 for 30 min, fluorescence intensity of 2,7-dichlorodihydrofluorescein (DCF) was determined to measure cellular ROS levels, and the alterations in ΔΨm were analyzed by flow cytometric FACscaning (Becton Dickinson, USA).
Statistical analysis
Results were expressed as mean ± SD and analyzed using ANOVA, using a significance level of P < 0.05.
Results
Combined effects of Bcl-2 siRNA and ATRA on HL-60 cells
To investigate whether Bcl-2 siRNA has the potential to sensitize HL-60 cells to ATRA, a combination treatment with Bcl-2 siRNA and ATRA was studied. The cells were exposed to Bcl-2 siRNA, followed by ATRA. Compared to the control at 24, 48 and 72 h, the viability of the cells was effectively reduced with Bcl-2 siRNA + ATRA, G3139 and Bcl-2 siRNA treatments (Table1). There was no difference on the viability of the cells among the control, GsiRNA and ATRA alone. Compared to treatments with the G3139 group, the viability of the cells of the Bcl-2 siRNA+ATRA and Bcl-2 siRNA groups was effectively reduced. The viability of the cells was significantly reduced by combining Bcl-2 siRNA and ATRA.
OD values after transfection with Bcl-2 siRNA using the MTT assay (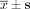 , n = 5).
, n = 5).
Effect of Bcl-2 siRNA on the Bcl-2 protein levels
As shown in Table 2, at 48 h after transfection, compared to control cells, Bcl-2 protein expression in the HL-60 cells was significantly reduced in all groups (P < 0.05) with the exception of the GsiRNA and ATRA groups. Compared to the G3139 group, only the siRNA group and siRNA plus ATRA group showed significantly reduced Bcl-2 protein expression. The control, GsiRNA, G3139 or ATRA showed no effect on Bcl-2 protein expression.
Bcl-2 protein levels after transfection with siRNA at 48 h (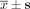 , n = 3).
, n = 3).
Intrinsic cellular ROS levels
At 48 h after transfection using a flow cytometry assay, ROS levels all obviously increased compared to the controls (Table 3). The ROS levels of he ATRA plus Bcl-2 siRNA group showed the highest level at about 2.5-fold over the control group. Compared with the G3139 group, both ATRA + siRNA and siRNA caused a significant increase in the cellular ROS level. After combination treatment with Bcl-2 siRNA plus ATRA, the cellular ROS level was 1.8 times more than ATRA group.
Cellular ROS level after transfection with siRNA at 48 h (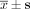 , n = 3).
, n = 3).
Cellular ΔΨm level after transfection with siRNA at 48 h (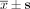 , n = 3).
, n = 3).
Bcl-2 siRNA disturbed the mitochondrial membrane potential in HL-60 cells
Compared with control group, at 48 h after transfection the mitochondrial membrane potential (ΔΨm) levels all markedly decreased in every group as measured by flow cytometry. The ΔΨm levels of ATRA plus Bcl-2 siRNA group was the greatest among all the groups. Compared with the G3139 group, either ATRA + siRNA or siRNA significantly decreased the cellular ΔΨm level. After combination treatment with Bcl-2 siRNA and ATRA, the cellular ΔΨm level was lower than either the ATRA or siRNA groups.
Discussion
Bcl-2 is a prominent member of the Bcl-2 family of proteins that regulate the induction of apoptotic cell death by a wide variety of stimuli. Increased tumor expression of Bcl-2 is considered to be one of the major determinants of resistance to chemotherapeutic drugs and radiotherapy[1-5,16,17]. Blocking of Bcl-2 expression and function may represent a relevant therapeutic strategy, and Bcl-2 antisense oligonucleotides have been successful in several preclinical and clinical studies. The current ongoing phase I~II clinical studies using antisense Bcl-2, known as G3139, aim at the treatment of patients with non-Hodgkin’s lymphoma, melanoma, and prostate cancer[4-7].
HL-60 cells have been previously shown to produce large amounts of Bcl-2. In this study, as expected, HL-60 cells transfected with Bcl-2 siRNA showed a significant decrease in cell proliferation and Bcl-2 expression as compared to control siRNA. Moreover, combined treatment with Bcl-2 siRNA plus ATRA enhanced the cellular ROS level and decreased the cellular ΔΨm level in the HL-60 cells. This additive pro-apoptotic effect also led to a concurrent reduction in cell number. There was no difference in the viability of the cells among groups treated with GsiRNA, G3139 or ATRA alone.
Our data indicate that down-regulation of the Bcl-2 protein by Bcl-2 siRNA increased the sensitivity of the HL-60 cells to ATRA, and that Bcl-2 siRNA may be useful for the treatment of tumors with over-expression of Bcl-2. This observation is in agreement with other findings, in which suppression of Bcl-2 can enhance sensitivity to chemotherapeutic drugs in tumor cells[18-20].
We previously have demonstrated that Bcl-2 antisense oligodeoxynucleotide enhanced drug-sensitivity in primary acute leukemia and chronic myeloid leukemia cells[1,2]. The combination of Bcl-2 down-regulation and ATRA produced a significant inhibitory effect on cell proliferation of the HL-60 cells compared to the two treatments given separately, indicating Bcl-2 knockdown caused a marked enhancement of ATRA-mediated inhibitory effect on cell proliferation.
Footnotes
The work was supported by a grant from the Key Subject Foundation of Overseas Chinese Affairs Office of the State Council (No. 51205002).
- Received April 25, 2007.
- Accepted January 7, 2008.
- Copyright © 2008 by Chinese Anti–Cancer Association











