keywords
Meningiomas occur in 1%~4% of primary intracranial tumors in the pediatric group, and is increasing in incidence with age[1]. Some authors have reported that meningioma is more prevalent among adult males, but there is no gender prevalence[1,2]. The accepted origin of meningiomas is from the arachnoid cells lining the meninges, or the choroid plexuses. Since Beckwith and Palmer[3] introduced the term ‘rhabdoid tumor’ in 1978 in reference to a subgroup of childhood malignant renal tumors, many tumors with a rhabdoid morphology have been reported in various sites, including the central nervous system. In 1998 Kepes et al.[4]and later Perry et al.[5] both described two series with clinically aggressive tumors that were a histologically distinct subgroup of malignant meningioma. They suggested the term rhabdoid meningioma which was adopted into the WHO classification (grade III) in 2000[6]. According to the current literature, most rhabdoid meningiomas behave aggressively and have a very poor prognoses[7].
Often rhabdoid meningiomas have almost exclusively been described in middle-aged, or elderly individuals[7-9]. Herein, we present a case of a girl with this extremely rare sub-type of malignant meningioma. Her clinical manifestations and treatment are reviewed, and discussed with reference to the literature.
Case Report
A 2-year-old girl presented with progressing headache and vomiting for 2 months, and with a 7-day-duration of seizures in her right hand. She was admitted to Tianjin Children’s Hospital by her parents. She had been delivered normally and was healthy otherwise. There was no history of trauma.
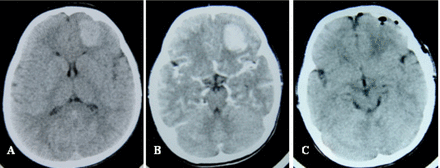
On examination, she was conscious and followed directions. Neurological examination revealed that there was moderate resistance to neck flexion and hemiparesis on her right side. Muscle power was grade 4 in her right limbs and grade 5 in the left limbs. No other abnormal neurological signs were elicited. No neurocutaneous markers were present. Preoperative non-contrast brain computed tomography (CT) revealed a slightly hyperdense, left frontal lesion, about 2.0×2.5×2.0 cm in size (Fig.1A). Contrast brain CT revealed enhancing left frontal mass with slightly surrounding edema (Fig.1B). The results of routine laboratory examinations (including haemoglobin, complete blood count, renal and liver function tests, and coagulation profile) were normal.
(A) Head axial-CT scan showing a solid lesion (2.0×2.5×2.0 cm) in the left frontal region. (B) Head axial-CT scan with contrast material showing the lesion markedly enhanced. (C) Post-operative head axial-CT scan showing the absence of the lesion.
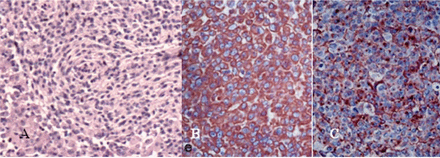
With the clinical impression of an intracranial tumor, a left fron-to-parietal craniotomy was performed, and a wellcircumscribed, grayish, friable and highly vascular mass penetrating the pia mater with a loose arachnoidal attachment was excised along with a 1 cm margin of adjacent dura. Gross total resection was achieved (Fig.1C). The tissues were stained with H&E (×100) with the tumor cells showing the typical cytologic features of rhabdoid cells, i.e., sheets of cells with variably abundant eosinophilic cytoplasm, eccentric nuclei, and hyaline paranuclear inclusions (Fig.2A). By immunohistochemical staining, glial fibrillary acidic protein was entirely negative, but vimentin and epithelial membrane antigen (EMA) were positive (Fig.2B, C). The pathological diagnosis was rhabdoid meningioma (grade III).
(A) Histological examination showing a rhabdoid meningioma (H&E, ×100). (B) Strong immunore-activity for vimentin (original magnification×400). (C) Strong immunoreactivity for EMA (original magnification×400).
The postoperative course was uneventful. No other therapies (chemotherapy and/or radiation therapy) were performed at the patient’s request. There was no recurrence of tumor in a follow-up examination after 2.5-years.
Discussion
Meningiomas in children
Meningioma is a tumor that arises from the meninges, the membranes that surround the brain and spinal cord. Risk factors for meningioma include: Radiation treatment, female hormones, and inherited nervous system disorders. The majority of meningioma cases are benign, although occasionally a meningioma can be malignant. Meningiomas occur mainly during the fifth decade of life, and account for 13.4 to 27.3% of all primary cerebral tumors[7]. Germano et al.[2] described an incidence of pediatric meningiomas at 1%~4.2%. Sixty to 80% of adult meningiomas occur in women, while in younger patients there is no sexual difference[2]. Meningioma is usually a very slowgrowing tumor, so signs and symptoms may be subtle and gradually worsen over a long period of time. Some people with a meningioma experience no signs and symptoms. Clinical presentation in children is with focal neurological deficits, seizures, and symptoms of increased intracranial pressure[2]. Our case was presented with headache, vomiting and seizures. The diagnosis of meningioma in this age group is difficult.
Rhabdoid meningiomas
Tumors with rhabdoid morphology were first described by Beckwith and Palmer[3] in 1978 as aggressive renal tumors in very young children. Subsequently, rhabdoid tumors and tumors with rhabdoid features have been described in many other organ systems, and all tend to have a poor outcome no matter what the histogenesis of the original tumor. Rhabdoid cells are plump, with abundant eosinophilic fibrillary to hyaline cytoplasm, and peripheral, vesicular, variably pleomorphic nuclei. Nucleoli are invariably present. While their morphology is well recognized in processed-biopsy material, their presence on a smear preparation can be quite startling. Although meningiomas are well-known entities with frequent histological variants, rhabdoid meningioma is an uncommon meningioma variant. It has been suggested that rhabdoid meningiomas are highly aggressive tumors and that the rhabdoid phenotype represents a marker of malignant transformation in meningiomas[5]. Therefore, it has been classified as a grade III malignancy in the 2000 WHO classification[6]. Martinez-Lage et al.[7] reported that rhabdoid meningiomas predominate in females, having a female: male ratio of 4:3. These tumors were found in sites where common meningiomas normally occur.
Histologic features and differential diagnosis of rhabdoid meningiomas
Histologically, rhabdoid meningiomas usually exhibit signs of anaplasia, high mitotic activity, and markedly increased MIB-1 (Ki-67) PI, a marker of cell proliferation[10]. The tumor cells have the typical cytologic features of rhabdoid cells, i.e., sheets of cells with variable abundant eosinophilic cytoplasm, eccentric nuclei, and hyaline; mitotic activity was 7 per 10 high power fields. By immunohistochemical staining, and glial fibrillary acidic protein are entirely negative, but the cells were abundantly vimentin-positive, whorled with a fibrillary cytoplasm[11]. EMA is variably positive in conventional and in rhabdoid meningiomas, but GFAP, S-100, desmin, and myogenin are universally negative[4-6].
One interesting feature of rhabdoid meningiomas is that in a significant number of cases, the rhabdoid cells appear after an initial recurrence. The exact causes of rhabdoid transformation from a benign meningioma are currently unknown[10]. Differential diagnosis of rhabdoid meningiomas have been established against other neoplasms that contain cells of rhabdoid morphology. Atypical teratoid/malignant rhabdoid tumors of the central nervous system are typically childhood neoplasms, associated with epithelial and mesenchymal components, with cells being positive for GFAP, actin, neruofilaments, and synaptophysin[7,12]. The diagnosis of gemistocytic astrocytomas is ruled out by the absence of GFAP[13]. In metastatic melanomas of rhabdoid cells that also have nuclear pseudo-inclusions, the nucleoli are more prominent, produce melanotic pigment, and are positive for S-100 and HMB-45[7,14]. Rhabdomyosarcomas are malignant mesenchymal neoplasms, which may also demonstrate rhabdoid cells, but these show characteristic cytoplasmic striae and are positive for striated muscle markers[7,15].
Rhabdoid meningioma treatment
Radical surgery (Simpson grade I) has been shown to significantly enhance the prognosis in atypical and malignant meningiomas. Some surgeons advocate preoperative emobolization to reduce intraoperative blood loss. However, with this technique there is the risk of embolic stroke and haemorrhage into a necrotic tumor[16]. Early conventional radiotherapy is recommended for atypical and anaplastic meningiomas irrespective of completeness of surgical excision, with numerous studies showing improved diseasefree survival. Median survival exceeding 5 years with combined radiochemotherapy has been reported. The majority of that survival benefit was probably due to radiotherapy as current chemotherapeutic regimens are not very effective against atypical/anaplastic meningiomas[17-19]. In the meantime, other therapeutic modalities are being increasingly explored.
Stereotactic radiosurgery (SRS) has been reported to be beneficial, even as a primary alternative to patients unable to undergo conventional surgery[20]. However, recent studies showed that there was no benefit for anaplastic tumors and poor control of atypical tumors[21]. It seems reasonable to use SRS as a postoperative for any tumor residuum, but not as a replacement for standard radiotherapy. Moreover, the use of SRS and radiotherapy significantly increases the incidence of radiation complications. Brachytherapy has been tried with 125I sources intraoperatively with mixed results[22]. Complications include a rate of radiation necrosis requiring further surgical resection of 13% in one series. Immunotherapy with interferon alpha, hyroxyurea and antiprostaglandins such as Mifepristone have been administered in unresectable tumors[23,24]. None have shown efficacy in treating atypical/anaplastic meningiomas. Recent interest in molecular therapies, including targeting tumor angiogenesis by inhibiting vascular endothelial growth factor (VEGF), or its receptor, shows future promise[25].
Pediatric rhabdoid meningiomas and outcome
The clinical features of 10 juvenile cases of rhabdoid meningiomas are summarized in Table 1. These patients’age ranged from 2 to 15 years (mean 10.8 years), with a female: male ratio of 7:3. Seven tumors were supra- and 3 infra-tentorial. Details of the completeness of tumor removal were known in 8 cases, and unknown in the others. Radiotherapy was given to 4 patients. After a mean follow-up of 86.6 months (except in 2 cases there was no follow-up), there was a recurrence in one child 4 months after the operation [31], and one patient died at 25 years after the first operation[32]. The other children who were followed were alive with stable disease, or with no evidence of recurrence. The outcome in pediatric patients with rhabdoid meningiomas seems to be better than that of their adult counterparts, in which there was a mortality rate of 49.2% after a mean follow-up period of 1 year[7]. Adjuvant therapy is essential in prolonging survival and management should at least consist of radical surgery and postoperative radiotherapy. The prognosis for these tumors seems to depend on their proliferative activity and the possibility of radical removal. Our case underwent total resection, and was without evidence of recurrence at the 30-month follow-up.
Review of publications concerning pediatric rhabdoid meningioma (<16 years).
Conclusion
Rhabdoid meningioma is an anaplastic, very rare subtype of malignant meningioma. It has menacing his-tology, early recurrence, and a likely poor outcome. Adjuvant therapy is essential in prolonging survival and management should at least consist of radical surgery and postoperative radiotherapy. The prognosis for rhabdoid meningiomas depends on their proliferative activity and the possibility of the radical removal. Juvenile cases of rhabdoid meningiomas appear to behave less aggressively than their adult counterparts.
Acknowledgements
The authors grateful acknowledge Prof. Ouyan Shi, Tianjin Medical University, for her constructive advice and Dr. Linsheng Zhao, Tianjin Children’s Hospital, for his assistance with reproduction of photographic images.
- Received October 24, 2007.
- Accepted December 24, 2007.
- Copyright © 2008 by Tianjin Medical University Cancer Institute & Hospital and Springer