Abstract
OBJECTIVE To investigate the expression of the nuclear transcription factor NF-κB, ER, HER2 and PCNA in breast cancers, and to study the relationship between activation of NF-κB and clinicopathologic parameters including the level of PCNA, ER, HER2, lymph node involvement, tumor size and histological grade (differentiation).
METHODS Sixty cases of human breast cancer tissues and adjacent non-neoplastic breast tissues were examined for NF-κB, HER2 and ER, as well as PCNA by immunohistochemical methods. In addition the clinicopathologic parameters of the patients including lymph node involvement, tumor size and histological grade (differentiation) were collected.
RESULTS The expression of NF-κB in the breast cancers and adjacent non-neoplastic breast tissue was 50.0% (30/60) and 40.0% (24/60) respectively, resulting in no significant difference (P>0,05). NF-κB and HER2 expression was positively correlated. whereas NF-κB and ER expression was negatively correlated. The NF-κB activation was 77.8% (14/ 18) in the breast cancers that were ER-/HER2+ a level significantly higher (P<0.001) in comparison to the other groups of patients. The expression of NF-κB in the low-differentiated group (grade III) was 57.1%, and in the moderate-differentiated group (grade II) was 50.3%, both of which were higher than the 35.7% found in the high-differentiated group (grade I). NF-κB activation in the cancers was significantly correlated with the histological grade (P<0.05), PCNA expression (P=0.003) and lymph node involvement and tumor size (P=0.03 and 0.002, respectively).
CONCLUSION NF-κB was activated abnormally in a portion of the breast cancers. The finding that NF-κB activation was positively correlated with FIER2 expression, the level of PCNA, tumor grade,size and lymph node involvement is in accord with the ability of NF-κB to promote cellular proliferation and migration, clearly identifies the protein as a hallmark for targeted dysregulation in oncogenesis. NF-κB may be a hopeful target for breast cancer therapy.
keywords
NF-κB is a transcription factor with activity that is tightly regulated at multiple levels.[1] Initially discovered and studied as a major activator of immune and inflammatory functions via its ability to induce expression of genes encoding cytokines, cytokine receptors, and cell-adhesion molecules, NF-κB has recently been implicated in the control of cellular proliferation and oncogenesis.[2] Ample evidence linking NF-κB activity to oncogenesis has accumulated in the past years. Previous breast cancer studies have proposed a potential role for NF-κB in the progression of hormone-dependent cancers to hormone independence. Constitutive activation of NF-κB was found in estrogen receptor-negative (ER-) breast cancer cell lines and poorly differentiated primary tumors. The proliferative and anti-apoptotic properties of activated NF-κB qualify this transcription factor as a key cellular regulatory molecule, potentially contributing to both normal and neoplastic phenotypes. Although elevated levels of this transcription factor in breast tumors have been reported,[3–5] the results show a strict association of activated NF-κB with distinctive subclasses of human breast cancers. Freund et al.[6] have highlighted the participation of NF-κB signaling in mammary epithelial cells, particularly in highly aggressive ER’ cells that overexpress the epidermal growth factor receptor (EGFR) family of receptors. Makino et al.[7] have reported that integrin-linked kinase (ILK), the crucial signal transducer in the integrin pathway, is involved in human epithelial growth factor receptor 2 (HER2)-mediated activation of NF-κB. Expression of HER2 increases ILK activity. Several clinical studies have shown that HER2+ breast cancers that are also ER+ have significantly lower ER than ER+ breast cancers that are HER-. Supporting these clinical observations, ER+ breast cancer cell lines engineered to overexpress HER2 remain ER+ but show marked reductions in their ER content. The role of NF-κB could be different in different tumor types. These concerns strongly indicate that further studies are necessary to dissect the roles of NF-κB in a variety of cancers, and to determine the applicability of inhibiting NF-κB as an adjuvant approach of standard cancer therapy. Here we report that NF-κB is aberrantly activated mainly in human breast cancers that overexpress the HER2 and have a low level of ER. NF-κB activation in breast cancer was significantly correlated with the histological grade and the level of proliferating cell nuclear antigen (PCNA)in cancer cells. These results suggest an important role for NF-κB in the pathogenesis of breast cancer and in potential treatment modalities.
MATERIALS AND METHODS
Breast carcinoma tissue samples
Tissue samples (60 cases) were surgically obtained from patients as part of treatment in Tianjin Cancer Hospital during the period of January 2002 to December 2004. The patients ranged in age from 28 to 69 years (mean 51). Among all patients, more than 20 lymph node dissections and biopsies were performed. Tissue samples were stained with hematoxylin and eosin and evaluated by a pathologist. The histological grade of the primary tumors was determined as recommended by the UICC. Then, the clinical and pathological records (histological types and lymph node status) were examined. Groups of the cancers were divided according to the presence or absence of lymph nodes with metastasis, histological grade and expression of ER. and HER2. Among the cases, 14 specimens were of a low malignancy grade (G I), 32 tumors were of a moderate malignancy grade classified as G II, and 14 cases were high-grade carcinomas (G III); 36 cases had positive lymphatic metastasis and 24 cases had no lymphatic metastasis, 18 cases were ER/HER2+, 18 cases were ER-HER2-, 12 cases were ER+/HER2+ and 12 cases were ER+/HER2- (Table 1).
Clinico -pathological data for 60 breast cancer cases
Immunohistochemical analysis
The breast cancer tissues were fixed in 10% formalin and embedded in paraffin, after which sections 5 pun-thick sections were cut and placed on glass slides. The slides were placed in xylene, passed through graded alcohols, rehydrated, treated with 3% H202 for 5 min, and washed in PBS. Immunostaining was performed using polyclonal antibodies against NF-κB (p50, Santa Cruz Biotechnology, Santa Cruz, CA, Sc-114), monoclonal mouse anti-human PCNA (PCNA, ZM-0213), with a biotinylated secondary antibody (Vectastain ABC kit; Vector Laboratories, Burlingame, CA) and horseradish peroxidase-conjugated streptavidin (Vector). Tissue sections were incubated with p50 antibody for 30 min. The slides were washed in PBS and incubated with a biotinylated secondary antibody, then incubated for 20 min with streptavidin-peroxidase conjugate. The colorimetric measurement was conducted using a diaminobenzidine (DAB) substrate. All steps were carried out at room temperature in a humidified chamber. The staining in dex was evaluated by 2 pathologists according to previously described criteria.[8] Quantitative measurements were expressed as the percentage of nuclear positivity for p50 or PCNA in the tumor cells. Ten microscopic fields per histological tumor sample were examined via light microscopy in 40x high-power fields. Sections stained with the various antibodies were then scored using a 3-point scale as follows: 0=negative (<5% of cells staining), l=weak staining (i.e. 5%~50% of cells showing weak to intermediate intensity staining in an appropriate subcellular distribution), 2=strong staining (i.e. >5% of cells showing very intense staining or >50% of cells showing weak to moderately intense staining, in an appropriate subcellular distribution). Scores were entered into a Microsoft Excel spreadsheet and simplified into either negative (score of 0) or positive (score of 1 or 2) categories.
In order to demonstrate the specificity of the immunoreactions, for negative controls the primary antibody was replaced by BSA or the secondary antibody alone. For positive controls lymphoma tissue samples were used.
Statistical analysis
The statistical analysis was performed with the chi-square and Spearman rank correlation tests using the SPSS software 12.0. A probability P value of less than 0.05 was considered statistically significant.
RESULTS
NF-κB activation in human breast cancer specimens
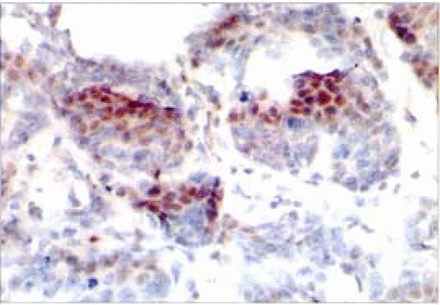
A key step in NF-κB activation is the degradation of its associated IKBS, which enables the released NF-κB dimers to move to the nucleus and transactivate target genes. The nuclear translocation of NF-κB p50 can be measured with the p50 antibody by immunostaining. The p50 associated generated a brownish-yellow signal in both the cytoplasm and nucleus of the cells (Fig. 1). Of note, although most cancer cells demonstrated nuclear p50 staining in some cases examined, nuclear staining was not observed in every individual malignant cell. NF-κB activation was defined as positive if the level of cells with nuclear staining in tissue was more than 25%. The expression of NF-κB in breast cancer tissue and adjacent non-neoplastic breast tissue was 50.0%(30/60) and 40.0%(24/60) respectively. The expression of NF-κB in breast cancer was not significantly different as compared to adjacent non-neoplastic breast tissue (P>0.05).
The p50 associated generated the brownish8888yellow signals in the nucleus of lhe bmast cancer cells.
NF-κB activation correlated with the status of expression of ER and HER2
To determine if the NF-κB activation in breast cancer tissues correlated with the status of expression of ER and HER2, all 60 breast cancer tissues in this study were divided into 4 groups, based on its status of ER and HER2. NF-κB was aberrantly activated significantly in human breast cancers that overexpress the HER2 and show a low level of ER. NF-κB activation was positive in 77.8% (14/18) of the breast cancer patients with ER/FIER2+ expression. Its activation was significantly higher (P<0.001) in comparison to the other groups of patients (Table 2).
NF-κB activation in 60 breast cancer cases with different status of ER and HER2 expression
NF-κB activation and ctinlcopathologic factors in breast cancers
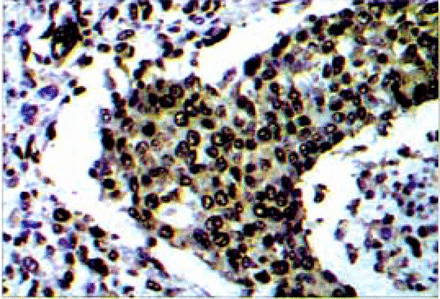
The association between NF-κB activation and clinicopathologic parameters including the level of PCNA, lymph node involvement, tumor size and histological grade (differentiation) is shown in Tables 1 and 3. PC-NA generated the brownish-yellow signals in the nucleus of the breast cancer cells (Fig.2). The level of PCNA in the cells was scored using a 3-point scale. NF-κB activation in breast cancer was significantly correlated with the level of PCNA (P=0.003). The positive rate of NF-κB activation in cases with lymph node involvement was higher than that in the group without lymph node involvement (P=0.030). The positive rate of NF-κB activation was correlated with tumor size (P=0.002, Table l). NF-κB activation in tumors of a size more than 2 cm was significantly higher than those which were in less than 2 cm. The positive rate of NF-κB activation in the low-differentiated group was 57.1%, and in moderate-differentiated group, 50.3%. These values were higher than the 35.7% found in the high-differentiated group. NF-κB activation in these cancers was significantly correlated with the histological grade (P<0.05, Table 3).
The PCNA associated generated the brownish-yellow signals in the nucleus of lhe breasl cancer cells.
NF-κB activation in 60 breast cancer cases with different histological grades
DISCUSSION
Activation of NF-κB correlated with the status of ER and HER2 in breast cancers
Human breast cancers are phenotypically heterogeneous and frequently pursue unpredictable clinical courses. Multiple and distinct molecular alterations presumably determine the diversity of histological patterns, pathological grades, and behaviors observed in these cancers. Clinicians rely on the expression of two important growth factor receptors, the nuclear ER and the membrane receptor tyrosine kinase, HER2 to classify human breast cancers into therapeutic and prognostic groups. Between 20% and 30% of human breast cancers express high levels of the HER2 protein, and at least 30% of ER-breast cancers contain overexpressed HER2. For the 60 patients in this study, there were 18 cases with ER/HER27 18 cases with ERY HER2’, 12 cases with ER7HER2+ and 12 cases with ER7HER2’. NF-κB was activated in 77.8% of the breast cancers which were ER/HER2+, an activation significantly higher (P<0.001) in comparison to the other groups of patients (Table 2).
A previous study showed that constitutive activation of NF-κB contributed to the progression of breast cancer to hormone-independent growth.[9] There was an association of NF-κB activity with ER-breast cancers, and presumably with the inactivity of this pathway in ER+ cancers. Since NF-κB regulates so many known survival and proliferation genes, it is not surprising that constitutive NF-κB activation has generally been implicated only with hormone independent (ER-) breast cancers. NF-κB activation rates in ER-/HER2-, ER+/HER2+ and ER+/HER2- groups were 33.3%, 41.7% and 41.7%, respectively. However, some cancer cells that demonstrated nuclear p50 staining in these 3 groups, located in front of the invasive of cancer cell in the mammary gland tissues. The expression of NF-κB and HER2 protein was positively correlated.
Biswas et al.[10] showed that the correlation of the NF-κB with HER2 expression could have resulted from the reported enhancement of the NF-κB pathway by HER2 RTK signaling. Zhou et al.[11] showed that HER2 constitutively stimulates NF-κB activity thrsough the PI-3K/Akt pathway. Makino et al.[7] identified a novel mechanism of HER2-mediated IKK activation, namely, up-regulation of IKKa and IKKb through ILK and Akt, a novel signal cascade: HER2/neu→PI-3K→ILK→Akt→IKKa/IKKb transcription resulting in NF-KB-induced cell proliferation and resistance to apoptosis.
The recent study by Zhou et al.[12) was the first to demonstrate that ER+ primary breast cancers can be prognostically subdivided according to their NF-κB activity, with increased p50 subunit DNA-binding activity appearing to be clinically more significant than increased p65 subunit DNA-binding activity. Their results showed that comparison of NF-κB DNA-binding activities in selected ER+ breast cancer cell line models are in keeping with other recent reports showing selective activation of NF-κB p50 in association with reduced tamoxifen sensitivity. These findings provide a rationale for further preclinical efforts aimed at evaluating the feasibility of therapeutically inhibiting NF-KB activity in order to improve efficacy of antiestrogen treatment in patients with high-risk hormone-dependent breast cancer. We found that NF-κB activation rates in both ER+/HER2+ and ER7HER2 groups was 41.7%, although some cell nuclear p50 staining in these 2 groups was located in front of the invasive cancer cells in the mammary gland tissues. It is suggested that therapeutically inhibiting NF-κB activity may not only be usefull for to cure ER-breast cancer treatment, but for ER+ breast cancer as well.
Activation of NF-κB in breast cancers and normal adjacent breast tissue
NF-κB is a transcription factor with activity that is tightly regulated at multiple levels. In most cell types, NF-κB complexes are normally sequestered in the cytoplasm as an inactive complex bound by an inhibitor known as IKB, until a cell is activated by a relevant stimulus.[13] Following cellular stimulation, IKB proteins become phosphorylated by the IKB kinase (IKK). This phosphorylation of IKB results in its ubiquitination and subsequent degradation by the 26S proteasome. The degradation of IKB proteins liberates NF-KB, allowing its translocation to the nucleus, which results in the blocking of cell-death pathways. Activation of NF-κB occurs in response to extracellular chemical stresses, various cytokines and growth stimuli. Lu et al.[14] showed that secretion of cytokines and growth factors is a general cause of constitutive NF-κB activation in cancer.
Our study which compared breast cancers with normal adjacent breast tissue suggest that expression of NF-κB in breast cancer was not significantly different compared to in adjacent non-neoplastic breast tissue (P>0.05). Diverse cell stimuli, e.g. tumor necrosis factor (TNF)a, CD40 ligand, interleukin (IL)-l, TRANCE, epidermal growth factor (EGF), phorbol esters, peroxides and ionizing radiation induce phosphorylation via activation of the IKB kinase complex, IKK. This results in subsequent proteasomal degradation of IKB inhibitory proteins and activation of NF-κB for nuclear translocation. There are high levels of cytokines in breast cancer tissue, which can activate NF-κB in adjacent non-neoplastic breast tissue.
NF-κB activation and clinicopathologic factors in breast cancer
Ample evidence linking NF-κB activity to oncogenesis has accumulated in the past years. A link between aberrant NF-κB activity and cancer was initially suggested by the identification of v-Rel, a viral homolog of c-Rel, as the transforming oncogene of an avian retrovirus that causes aggressive tumors in chickens. High levels of NF-κB were shown to be essential for the transformed phenotype. Constitutive nuclear NF-KB activity has emerged as a hallmark for many other human leukemias, lymphomas, and solid tumors. Furthermore, several oncoproteins, including Ha-Ras and Bcr-Abl, are known to activate NF-κB and rely on NF-κB to mediate their transforming activity.
Kim et al.[15] findings indicated that the activation of NF-κB in the mammary glands upon carcinogen treatment of rodents is an early event. In our study, we showed that the level of activation of NF-κB in breast cancer was significantly correlated with the histological grade of the tumors. The NF-κB activation rate in the low-differentiated group was 57.1%, and in moder-ate-differentiated group 50.3%, which was higher than the 35.7% found in the high-differentiated group. These results suggest that activation of NF-κB occurs early during neoplastic transformation of the breast cells, and becomes higher as the cancer progresses. In addition, the positive rate of NF-κB activation was correlated with tumor size (P=0.002, Table l). Helbig et al.[16] showed that NF-κB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. Recent studies have suggested that NF-κB regulates the expression of multiple genes involved in tumor spread and metastasis, including those encoding MMPs, IL-8, VEGF, and CXCR4. Evidence has accumulated showing that EMT represents an important in vitro correlate of late stage tumor progression. NF-κB is essential for epithelialmesenchymal transition and metastasis in a model of breast cancer progression.[17] In our study, we showed that the level of activation of NF-κB in breast cancer was significantly correlated with lymph node involvement. The level of NF-κB expression in the tumor with lymph node involvement was higher than those without lymph node involvement (P=0.030).
Activation of NF-κB is correlated with expression of PCNA in breast cancer cells
There is mounting evidence for NF-κB mediating signals that induce cell proliferation, and constitutive NF-KB activity has been shown to correlated with cancer cell proliferation. PCNA, an auxiliary polymerase protein, plays an important role in DNA synthesis. Its expression represents an important marker that reflects proliferation ability of carcinomas.[18],[19] The constitutive activation of NF-κB has been linked with the etiology and progression of hormone-independent (ER ) breast cancers, in part due to its transcriptional stimulation of genes that direct cell proliferation and invasion such as cyclin D1 and urokinase-type plasminogen activator (uPA). The level of cyclin D1 in cells is significantly correlated with PCNA expression. Our immunohistochemical staining showed that the level of PCNA expression in breast cancer cells was significantly correlated with the level of activation of NF-κB. These results suggests that activation of NF-κB in breast cancer cells promotes their proliferation.
In conclusion, NF-κB was activated abnormally in a portion of the total breast cancers. Activation of NF-KB was positively correlated with HER2 expression and the level of PCNA, and was negative correlated with ER expression. The capacity of NF-κB to promote cell proliferation and migration while suppressing differentiation and apoptosis clearly identifies the protein as a hallmark for targeted dysregulation in oncogenesis. NF-κB may be a hopeful therapeutic target for breast cancer.
Footnotes
This work was supported by the National Natural Science Foundation of China (No. 30471962).
- Received December 20, 2005.
- Accepted January 10, 2006.
- Copyright © 2006 by Tianjin Medical University Cancer Institute & Hospital and Springer