Abstract
Objective: To establish a new classification of biliary tumor thrombus (BTT).
Methods: Overall survival of patients with BTT was first used to determine whether it correlated with current hepatocellular carcinoma staging systems. Univariate and multivariate analyses were used to determine factors affecting the overall survival (OS) to form the basis of our new classification for BTT.
Results: All 6 international staging systems showed overlapping survival curves. Univariate followed by multivariate analyses showed that total bilirubin and intrahepatic/extrahepatic BTT were significant risk factors of OS. Based on these data, a new BTT classification was defined as: Type I: intrahepatic BTT; and Type II: extrahepatic BTT involving a common bile duct or common hepatic duct. Type I was further subdivided into type Ia: BTT involving a second-order intrahepatic duct or above, and type Ib: BTT involving a first-order intrahepatic duct. Type II was further subdivided into type IIa and type IIb using a cut-off total bilirubin (TB) > 300 μmol/L. The numbers (percentages) of patients with types I and II BTT were 69 (34.2%) and 133 (65.8%), respectively. The median OS of type I patients was significantly higher than that of type II patients (37.5 months vs. 23.2 months; P = 0.002). Using subgroup analyses, OS outcomes were significantly different between the subgroups of type IIb and type IIa, although there was no significant difference between the type Ia and type Ib subgroups (P = 0.07).
Conclusions: A new BTT classification was established to predict prognoses of HCC patients with BTT who underwent liver resection.
keywords
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common neoplasm and the third leading cause of cancer-related mortality worldwide1. Although HCC has a strong tendency to involve vascular structures, biliary tumor thrombus (BTT) is also a well-known and important clinical presentation. The incidence of BTT has been reported to be 1%–9%2–4. Because jaundice presents in a significant percentage of HCC patients with BTT at the time of diagnosis, misdiagnosis as progressive liver failure or diffuse tumor infiltration of the liver parenchyma often occurs. With improvements in imaging techniques, the diagnosis of BTT is becoming more straightforward and accurate5. Clinically, it is important to identify this group of patients because surgical treatment can be beneficial6. A Korean-Japanese multicenter study2 showed a 5-year survival of up to 43.6%, indicating that an aggressive surgical approach is the choice of treatment in selected BTT patients.
The current international HCC staging systems that are commonly used, including the TNM classifcation7, Cancer of the Liver Italian Program (CLIP) score8, the Japan Integrated Staging (JIS) scoring system9, and the Barcelona Clinic Liver Cancer (BCLC) classification10, only consider vascular invasion to be an important prognostic factor, but do not include BTT. Several studies have classified BTT types11,12, but all these classifications are based only on anatomy, which is inadequate to predict the prognosis and guide management. A specific classification that includes clinical parameters in addition to anatomy is urgently needed. This study therefore established a new classification to better stratify and refine the types of BTT, with the aim of improving prognostic predictions and guiding clinical management.
Materials and methods
Patients
A multicenter retrospective study was conducted on a prospectively maintained clinical and pathological database of HCC patients. Consecutive HCC patients with BTT who underwent liver resection at the Eastern Hepatobiliary Surgery Hospital (EHBH), Fujian Provincial Hospital, West China Hospital of Sichuan University, First Affiliated Hospital of Fujian Medical University, and Zhongshan Hospital of Xiamen University were enrolled in this study. The presence of BTT was diagnosed using preoperative radiological imaging (ERCP/CT/MRI/ultrasound) and was subsequently confirmed by postoperative histopathological studies3,5. The inclusion criteria were as follows: (1) age between 18–75 years with an Eastern Cooperative Oncology Group performance status score of 0 or 1; (2) resectable HCC, which was defined as either a single tumor <10 cm in diameter or multiple HCCs confined to 1 hemi-liver; (3) macroscopic BTT on preoperative medical imaging; (4) no extrahepatic or distant metastases; and (5) no other associated malignancies. The present study was approved by the Research Ethics Committees of all participating hospitals. Informed consent was obtained from all patients prior to treatment and for their data to be used for research purposes.
All patients underwent routine preoperative imaging, including abdominal ultrasonography, contrast-enhanced magnetic resonance imaging (MRI) and/or computed tomography (CT) of the abdomen and chest. Routine serological examinations included complete blood analyses, liver and renal function, hepatitis B and C serology, HBV DNA load, and serum α-fetoprotein (AFP) level tests.
Surgical procedures
All the preoperatively and intraoperatively detected lesions were resected. If the BTT was located within the planned hepatic resection region, the BTT was removed en bloc with the resected part of the liver. If the BTT extended to the common bile duct, thrombectomy with extraction of the BTT from the opened stump of the right/left hepatic duct was performed in combination with cholangio-jejunostomy. Intraoperative ultrasound was used to confirm that no residual tumor or BTT was left behind.
Follow-up
All patients were followed-up once every month for the first 3 months postoperatively and then once every 3 months thereafter. The follow-up examinations included routine blood tests, liver function tests, AFP tests, chest radiography, abdominal ultrasound, and CT/MRI. HCC recurrence was diagnosed based on typical imaging features on CT and/or MRI, with or without abnormal AFP levels. If HCC recurrence was diagnosed, appropriate treatments including further liver re-resection, local ablative therapy, and/or regional or systematic therapy were performed.
Statistical analysis
Categorical variables were analyzed using the χ2 test or Fisher’s exact test, and continuous variables are expressed as the mean ± standard deviation (SD) and were compared using the Mann-Whitney test. Survival curves of recurrence-free survival and OS were obtained using the Kaplan-Meier method and compared using the log-rank test. Median survival times and their 95% confidence intervals (CIs) were reported. Factors that were found to be significantly (P < 0.05) associated with survival using univariate analysis and any imbalanced factors between groups were entered into a Cox A proportional hazards model to test for significant effects while simultaneously adjusting for multiple factors. For all tests, a P < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS statistical software for Windows, version 24.0 (Chicago, IL, USA).
Results
Patient characteristics
This study included 202 consecutive HCC patients with BTT who underwent liver resection from June 2009 to December 2017 at the Eastern Hepatobiliary Surgery Hospital, Fujian Provincial Hospital, West China Hospital of Sichuan University, First Affiliated Hospital of Fujian Medical University, and Zhongshan Hospital of Xiamen University. Among the 202 patients, 41 (20.3%) patients had vascular invasion, and 10 (5.0%) patients had lymph node metastases. The numbers (percentages) of patients with intrahepatic and extrahepatic BTT were 146 (34.2%) and 133 (65.8%), respectively. At a median follow-up of 28.4 months (range, 13–112 months), 141 patients (69.8%) died.
Predictive accuracy of the commonly used international staging systems
Kaplan-Meier curves were generated for the BCLC staging, TNM staging, CLIP, JIS, Ueda, and Japanese B-classification systems (Figure 1). The survival outcomes are also shown in Table 1. All 6 systems showed overlapping survival curves, with no clearly defined prognostic strata.
Kaplan-Meier curves of 6 systems which all showed overlapping survival curves. (A) Kaplan-Meier curves of the Barcelona Clinic Liver Cancer staging. (B) Kaplan-Meier curves of the TNM staging. (C) Kaplan-Meier curves of the CLIP. (D) Kaplan-Meier curves of the JIS. (E) Kaplan-Meier curves of the Ueda classification system. (F) Kaplan-Meier curves of the Japanese B-classification system. All 6 systems showed overlapping survival curves.
Survival outcomes of the 6 staging systems
Multivariate analysis showed that TB < 300 μmol/L hazard ratio (HR: 2.075; 95% CI; 1.210–3.559; P = 0.008) and intrahepatic/extrahepatic BTT (HR: 1.600; 95% CI, 1.097–2.332, P = 0.015) were significant risk factors for OS (Table 2).
Univariate analysis and multivariate analysis of overall survival
Survival analysis of our new classification of BTT
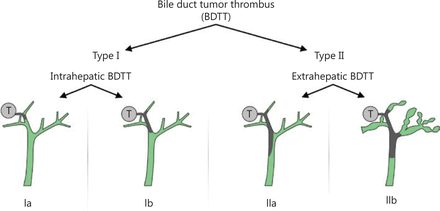
HCC patients with BTT were divided into 2 grades based on the 2 significant risk factors for OS of TB and intrahepatic/extrahepatic BTT: type I, intrahepatic BTT; type II, extrahepatic BTT involving the common bile duct or common hepatic duct. Type I was further subdivided: type Ia, BTT involving the second-order intrahepatic duct or above; and type Ib, BTT involving the first-order intrahepatic duct. Based on the need for preoperative biliary drainage for patients with TB > 3,000 μmol/L, type II was further subdivided into type IIa and type IIb (Figure 2). The clinicopathological features of the 2 types of BTT patients are shown in Table 3.
Patterns of biliary tumor thrombus type systems. T: hepatic tumor.
The clinicopathological features of the 2 types of biliary tumor thrombus patients
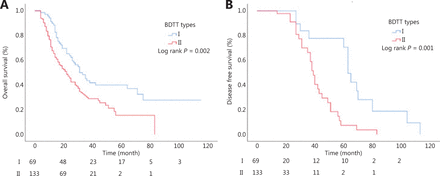
The median OS of type I patients was significantly longer than that of type II patients (37.5 months vs. 23.2 months; P = 0.002) (Figure 3A). The median DFS of type I patients was significantly longer than that of type II patients (21.2 months vs. 14.0 months, P = 0.001) (Figure 3B).
Overall survival according to different biliary tumor thrombus types. (A) The median OS of type I and type II. (B) The median DFS of type I and type II.
Subgroup analysis
The median OS of the type IIb subgroup was significantly shorter than that of the type IIa patients (17.4 months vs. 23.9 months; P = 0.023) (Figure 4A). Furthermore, if type IIb patients underwent preoperative biliary drainage, the median OS was prolonged from 11.7 months to 22.9 months. There was no significant difference in OS between the type Ia and type Ib BTT groups (44.4 months vs. 30.4 months; P = 0.070) (Figure 4B). The recurrence and survival outcomes of the patients with different subtypes of BTT are shown in Table 4.
Kaplan-Meier curves of the subtypes of the new biliary tumor thrombus types. (A) The median OS of type Ia and type Ib. (B) The median OS of type IIa and type IIb.
Survival outcomes of the 2 biliary tumor thrombus subtypes
Discussion
To the best of our knowledge, our study is the first to classify BTT in HCC patients based on both anatomy and TB levels. Multivariate analysis confirmed the new BTT classification is a significant risk factor for OS. This new classification divided BTT into 2 types, which were easy to differentiate by radiological imaging. The new BTT classification is practical and can easily be used in clinical practice. Using this new classification, en bloc resection is recommended for HCC patients with type Ia and type Ib BTT. However, for type IIa patients, liver resection combined with thrombectomy should be considered. For type IIb patients, biliary drainage is recommended before surgery.
The incidence of hyperbilirubinemia has been reported to be 19%–40% in HCC patients at the time of diagnosis13. Cirrhosis and/or extensive hepatic parenchymal destruction by tumors explains jaundice in the majority of patients, who have a very poor prognosis. With improvements in medical imaging methods14, the diagnosis of HCC with BTT can now be made easily. In our data, 92 HCC patients with BTT (45.5%) presented with obstructive jaundice. Identification of this group of patients is clinically important because surgical treatment is usually beneficial15. Many studies have confirmed2,16,17 that hepatic resection with removal of the BTT is the treatment of choice in selected BTT patients with resectable hepatic tumors, even for those patients presenting with obstructive jaundice.
The low incidence of BTT has led to an insufficient understanding of this complication, and the commonly used international HCC staging systems rarely consider BTT to be an important prognostic factor. The currently available typing methods for BTT are therefore inadequate for this complex problem. The TNM, JIS, and CLIP staging systems all showed overlapping survival curves using statistical analysis, and they are not refined enough to guide treatment or predict prognosis when HCC patients with BTT are still at a resectable stage. The Japanese B-classification and Ueda typing guidelines are the most widely used typing methods for BTT, but they consider only the extent of BTT, without any consideration of the degree of hyperbilirubinemia, which is a very common presentation of BTT, as well as an independent prognostic factor for OS. Thus, our staging system is clinically better for HCC patients with BTT.
Type II BTT patients accounted for approximately 2/3 of all patients in our series, and the treatment of these patients was more complex than that of type I patients. Many clinical problems have not been solved, including when preoperative biliary drainage should be used and how low the TB level should be before liver resection is conducted. Our data showed that TB < 300 μmol/L was an independent prognostic factor of OS, and for patients with TB levels higher than 300 μmol/L, the median OS was significantly prolonged with preoperative biliary drainage. Our study suggested that HCC patients with type IIb BTT should undergo preoperative biliary drainage.
This study had limitations. First, this was a retrospective study with inherent bias. Second, the sample size was small. Third, this study was performed in China with a high percentage of HBV-related HCC patients. Whether the results of this study can be extrapolated to other causes of HCC is unknown. Fourth, all patients in the study underwent liver resection, so, this typing method cannot be used in patients treated without surgery.
In conclusion, a new BTT classification was established. It can be used to predict the prognoses of HCC patients with BTT who underwent partial hepatectomy. This BTT classification is better for predicting prognosis and guiding treatment than other commonly used staging systems. Furthermore, this new classification can also be used to supplement HCC classification or scoring systems.
Grant support
This work was supported by grants from Youth Project of Shanghai Municipal Health Commission (Grant No. 20184Y0153), the Shanghai Young Physicians Training Program (Grant No. 2018-15), the Medical Innovation Project of Health and Family Planning Commission of Fujian Province (Grant No. 2018-CX-8), the Key Project of Natural Science Foundation of China (Grant No. 81730097), a grant from the Science Fund for Creative Research Groups (Grant No. 81521091), and the National Natural Science Foundation of China (Grant Nos. 81602523 and 81702335).
Footnotes
↵*These authors contributed equally to this work.
Conflict of interest statement No potential conflicts of interest are disclosed.
- Received May 2, 2020.
- Accepted September 16, 2020.
- Copyright: © 2021, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.