On March 5th, a novel immunosuppressive molecule, Siglec-15, was reported in Nature Medicine by Professor Lieping Chen1. Siglec-15 was reported in a wide variety of tumors. The molecule is not a simple replica of PD-1/PD-L1. Rather, the expressions of Siglec-15 and PD-L1 are mutually exclusive1, suggesting that Siglec-15 antibodies may be effective on tumors that are not responsive to anti-PD-1/PD-L1 therapy. Siglec-15 potentially serves as a complementary therapeutic target and offers alternative treatments for many anti-PD-1/PD-L1 therapy unresponsive patients. This discovery generated a huge response. Moreover, after the specific anti-Siglec-15 monoclonal antibody (α-S15) achieved encouraging results in various mouse tumor models, a first-in-human phase I clinical trial is ongoing to test the effect of a humanized monoclonal antibody (NC318) to Siglec-15 in solid tumors (NCT03665285). Furthermore, since the Siglec-15 pathway is one of the pathways of tumor immune escape, therapies that target it are also a part of the normalization cancer immunotherapy proposed by Professor Chen2.
Cancer immunotherapy: from enhancement to normalization
Immunotherapy has become the fourth main mode of cancer treatment after conventional therapies including surgery, radiotherapy, and chemotherapy. Immunotherapy is regarded as the most promising of these treatment modes because of its limited side effects, high anti-tumor activity, and wide indications for use. On October 1, 2018, the Nobel Prize in Physiology or Medicine was awarded to James P. Alison and Tasuku Honjo for the discovery of immune checkpoints. On the eve of Nobel Prize announcement, as one of the leading researchers of immune checkpoints, Professor Lieping Chen proposed the latest direction of immunotherapy—normalization cancer immunotherapy—in the journal Cell2.
The concept of normalization cancer immunotherapy emphasizes the importance of identifying specific defects or dysfunctions of immune responses during tumor progression and correspondingly developing specific strategies to correct these defects to restore normal anti-tumor immunity.
Immune responses are always vital in anti-tumor treatment. Therefore, people have long thought of augmenting the human immune activation mechanism that eliminates viruses and bacteria to increase the potency or degree of the anti-tumor immune response and eventually kill the target tumor. Some methods of this strategy, including antibody targeted therapy, adoptive immune cell therapy, genetically engineered T cells, tumor vaccines3, cytokines4, and more are designed to activate and enhance the immune response. The approach has been termed "immunity enhanced therapy." However, this strategy often fails to achieve the desired objective response rate. Moreover, without specifically targeting the tumor microenvironment, these strategies may cause the widespread activation of the immune system, thereby greatly increasing the incidence of immune-related adverse events (irAEs), and even leading to autoimmune diseases5,6. Meanwhile, tumors actively use various strategies to delay, alter, or even prevent anti-tumor immunity. Those strategies, collectively referred to as "immune escape mechanisms", have prevented the immune system from effectively suppressing tumor growth, which can ultimately lead to cancer progression.
In the last decade, a new method of immunotherapy has emerged, represented by the inhibitors of immune checkpoint PD-1/PD-L15,7,8, which precisely target the tumor immune escape mechanism. When the PD-1/PD-L1 pathway is activated in the tumor microenvironment, the anti-tumor immune response of effector T cells is suppressed. Therapies that block this pathway effectively improve anti-tumor immune responses against multiple tumor types. The United States Food and Drug Administration has approved 15 anti-PD-1/PD-L1 medicines for cancer indications. Some of these have also been added to the first-line treatment of certain cancers, such as lung cancer9. Anti-PD-1/PD-L1 medicines often result in an objective remission rate that is as good as, or better than, the effect of traditional immunotherapy, and features a lower incidence of irAE. This increasingly specific tumor-targeting toxicity is because this therapy targets tumor-specific immune escape mechanisms in the tumor microenvironment (TME) and corrects tumor-induced immune defects. This is what Professor Chen refers to as normalization cancer immunotherapy.
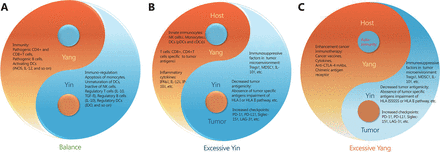
Normalization cancer immunotherapy conforms with the laws of nature. The normal function of the human body depends on the balance and stability of various systems, and the immune system is no exception. Since ancient times, traditional Chinese medicine has always emphasized the concept of the balance of Yin and Yang (Figure 1), which is comparable to the homeostasis concept in modern medicine. In other words, the human body is viewed as a unified whole in which a slight alteration in one part can affect the whole. The balanced growth and decline in the transformation of Yin and Yang maintains coordination, which is neither excessive abundant nor exhausted, and which results in a state of harmonization. Individuals with balanced Yin and Yang produces are healthy and in good spirits. Those with an imbalance are inclined to be sick, prematurely aged, and can even have a shortened lifespan. As Chinese traditional medicine asserts, excessively strengthening or excessively weakening a certain function of the body will inevitably lead to imbalance within the body, and this imbalance will cause damage to the body. Similarly, in the treatment of cancer, early traditional immunotherapy can actually attack the tumor by over-activating the immune response, but the effect of this immune activation often spreads throughout the body, and normal tissues and organs can also come under attack. The human body cannot withstand such a strong immune response, and side effects of autoimmune damage occur, which is an important reason for limiting the widespread use of traditional immunotherapy.
The balance of Yin and Yang in immunity. (A) The balance of immunity in healthy people. (B) After tumorigenesis, tumor uses various immunosuppressive mechanisms to escape from immune attack. (C) In conventional immunotherapies, excessive immune enhancement can cause autoimmune diseases.
Therefore, the concept of normalization cancer immunotherapy has arisen at the right time. It emphasizes correcting the tumor-induced immune over-suppression state and not excessively activating the immune system, so that the immune system is maintained at the correct intensity that is effective in maintaining the balance in the body while killing the tumor.
The transformation of tumor immunotherapy strategy from immune enhancement to immune normalization was elucidated by Professor Chen in the September 2018 Cell review paper titled A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization2. The review described the three principles of normalization cancer immunotherapy—targeting a tumor-induced immune escape mechanism, selectively modulating immunity in the TME, and resetting immunity in the TME. These three principles also state the reason why normalization cancer immunotherapy can reduce the incidence of immune adverse events while improving the efficacy of anti-tumor treatment.
Anti-PD-1/PD-L1 treatment is currently the best known and most clinically effective method for normalization cancer immunotherapy, but it is only effective in 20% to 30% of human solid tumors10-12. The low efficiency indicates that there are other potential immunosuppressive pathways, such as the newly discovered Siglec-15 pathway, as well as the FGL1-LAG-3 (Fibrinogen-like protein 1, FGL1) pathway also reported by Professor Chen13. They are all strategies for tumor immune escape and are potential targets for normalization cancer immunotherapy.
TCAA: a new member in the arsenal of tumor basic and translational research
The discovery of the new immunosuppressive checkpoint Siglec-15 relies on a powerful high-throughput in vitro functional screening system constructed by Professor Chen, which is termed the genome-scale T cell activity array (TCAA). It can recognize cell surface molecules that regulate T cell activity in vitro1, and identify new targets for immunotherapy. The discovery of the ligand for LAG-3 involved the construction and use of a similar tool called the genome-scale receptor array (GSRA). Use of the GSRA was instrumental in the discovery of FGL1-LAG-3 as a new immunosuppressive pathway13. The TCAA contains more than 6, 500 human genes encoding more than 90% of the transmembrane proteins in the human genome.
This high-throughput screening tool was achieved by constructing a cell line in vitro in which individual genes are transiently expressed in the array. In brief, a 293T-based artificial antigen-presenting cell (aAPC) line was designed that expresses a membrane-associated anti-human CD3 (OKT3) single-chain variable fragment (scFv) for T cell receptor activation, and several transmembrane signal transduction genes to promote membrane protein expression13. The Jurkat T cell line was utilized to detect the function of the target gene and its effect on T cell activity. A stable expression of green fluorescent protein (GFP) reporter gene driven by nuclear factor-kappa B or NFAT response elements was established in the Jurkat T cells. Comparison with the blank control group allows the GFP signal strength to be used to identify the effect on T cell activity of the membrane protein expressed on aAPCs. Genes that continuously inhibit or enhance GFP signals can be selected after multiple rounds of TCAA screening and subsequently verified. The genes that have been identified through TCAA to affect T cell activity include some that are already reported as co-stimulatory molecules, apoptosis-related genes, or co-suppressor molecules, which also validate the accuracy of the TCAA system.
Professor Chen used this screening system to demonstrate that Siglec-15 can continuously inhibit T cell activity and to indicate anti-Siglec-15 therapy may meet the main features of normalization cancer immunotherapy2. Based on these attributes, Siglec-15 was selected for further study. Subsequent comprehensive analyses in vivo and in vitro confirmed the immunosuppressive function of Siglec-15 and suggested that Siglec-15 could be used as a potential target for the tumor immune normalization strategy. The sensitivity and reliability of this powerful tool are good, and it is efficient and accurate. Prior to this, examinations for proteins with a certain function, protein-protein interactions, or ligand-molecule interactions generally used are co-immunoprecipitation, pulldown, and CRISPR screening. Compared with TCAA, these technologies are inefficient and have lower accuracy. They suffer from too many false positive results, and so the target molecules may not be screened out. TCAA is more objective and accurate. In the future, cell-display may become the mainstream receptor ligand interaction screening tool.
In future research, we may also use this tool to explore the mechanism behind tumor development and search for potential therapeutic targets that are truly efficacious against tumors.
Discovery of the novel Siglec-15 immune suppressor and its vital role as an immune normalization target
In addition to the PD-1/PD-L1 pathway, the up-regulation of other inhibitory molecules, cytokines, metabolites, and inhibitory immune cells in the TME, as well as the down-regulation of many immune-stimulatory molecules and immunocompetent cells, may become new targets for addressing immunosuppression14,15. Siglec-15 is a promising target.
After screening the Siglec-15 immunosuppressive molecule using TCAA, Professor Chen conducted a series of rigorous and comprehensive experiments to verify the role of Siglec-15 in tumors. The Siglecs are a family of sialic-acid-binding immunoglobulin-like lectins. They can promote cell-cell or cell-pathogen interactions by recognizing sugar chain structures containing sialic acid. Thus, they have an important regulatory role in innate and adaptive immunity16. Siglec-15 is a member of the Siglec family that encodes a very short extracellular domain17. Although the role of Siglec-15 in osteoclast differentiation and bone remodeling has been reported18, its immune function remains largely unknown. Protein sequence analysis showed that the Siglec-15 extracellular domain comprises an immunoglobulin variable region (IgV) and a type 2 constant region (IgC2), and has more than 30% homology with the B7 gene family, indicating a close relationship between Siglec-15 and the B7 gene family. Siglec-15 may have immunomodulatory functions that are similar to members of the B7 family.
The expression of Siglec-15 mRNA is rare in most normal human tissues and various immune cell subpopulations. Siglec-15 protein is expressed mainly on macrophages. TCCA database analysis revealed upregulated Siglec-15 mRNA expression in various tumors including colon cancer, endometrioid carcinoma, thyroid cancer, bladder cancer, kidney cancer, lung cancer, and liver cancer. Immunohistochemical analysis of 241 non-small cell lung cancer samples showed that Siglec-15 was up-regulated on tumor cells, tumor-associated stromal cells, and tumor-infiltrating macrophages/myeloid cells. Siglec-15 knock-out mice do not develop autoimmune diseases or other symptoms. A series of in vitro and in vivo experiments revealed that macrophage/myeloid-derived Siglec-15 can inhibit the immune response of antigen-specific T cells by regulating cell growth and promoting the survival and differentiation of inhibitory macrophages. All of these findings are consistent with the characteristics of targets for normalization cancer immunotherapy. The researchers further demonstrated that Siglec-15 is a potential target for tumor immune normalization strategies in mouse tumor models. B16-GMCSF tumor cells were introduced into Siglec-15 knock-out mice; the result was decreased tumor growth and prolonged survival compared with wild-type mice. In tumors of the knock-out mice, the infiltration of CD8+T cells and natural killer (NK) cells was also significantly increased, and the production of cytokines, such as interferon-gamma of CD8+T cells was also significantly improved. In addition, Professor Chen designed an anti-Siglec-15 antibody that was used in a variety of mouse tumor models. Interestingly, α-S15 can block the immunosuppressive effects of macrophage/myeloid cell-derived Siglec-15 to improve anti-tumor immune response in vivo.
Immunohistochemistry also revealed the lack of correlation between Siglec-15 and PD-L1 expression. The expression of Siglec-15 is mutually exclusive to PD-L1, suggesting that these two molecules may mediate immune-suppressions through completely different mechanisms. When co-cultured with wild-type bone marrow-derived macrophage (BMDM), α-S15 can promote the response of PD-1 knock-out T cells. This result confirmed that Siglec-15 can suppress the immune response in a manner that is independent of the PD-1/PD-L1 pathway. It is envisaged that the therapy by targeting Siglec-15 may become beneficial for many anti-PD-1/PD-L1 therapy unresponsive patients.
In general, the discovery of the new immunosuppressive molecule Siglec-15 is of great significance for several reasons, which are listed below:
(1) The discovery of Siglec-15 can partially explain why anti-PD-1/PD-L1 therapy is only effective in 20% to 30% of human solid tumors. The PD-1/PD-L1 pathway is only one of the many mechanisms of tumor immune escape. In patients with other mechanisms, such as the Siglec-15 pathway, the effect of anti-PD-1/PD-L1 therapy may be less effective.
(2) Anti-Siglec-15 may be a potential therapy for those who do not respond to anti-PD-1/PD-L1 therapy and may become a powerful supplement for PD-1/PD-L1 inhibitors. Siglec-15 and PD-1 have mutually exclusive expression patterns, suggesting that the Siglec-15 pathway may be the main inhibitory mechanism in some conditions. Targeting Siglec-15 is likely to produce promising therapeutic effects.
(3) Anti-Siglec-15 antibodies combined with anti-PD-1/PD-L1 antibodies can have a synergistic effect. This synergistic effect has been confirmed in mouse models, where the effect of the combination of the two monoclonal antibodies is stronger than their respective effects. The combined use of the two treatments in human tumors may perhaps also have this synergistic effect.
(4) Siglec-15 can be used as a biomarker for the prediction of anti-PD-1/PD-L1 therapeutic effects. The high expression of Siglec-15 often indicates that the Siglec-15 immunosuppressive pathway plays a pivotal role in the TME of some patients. The PD-1/PD-L1 pathway may not be the main cause of immunosuppression, and so Siglec-15 can continue to exert its inhibitory effect even if the PD-1/PD-L1 pathway is blocked.
(5) The discovery of Siglec-15 and the subsequent confirmation of its important immunosuppressive effect in tumor development attracted the attention of more scientists and pharmaceutical companies, and brought confidence and new ideas to scientists to explore more immunosuppressive checkpoints.
Highlights of this study include the following:
(1) The design of this research experiment is comprehensive and rigorous. From database to immunohistochemistry, from gene knock-out mouse models to the effect of monoclonal antibody in tumor-bearing mice, multi-directional verification of the immunosuppressive effect of Siglec-15 were convincingly performed.
(2) Animal experiments suggest that Siglec-15 inhibition may not produce serious immune-related toxicity. With this assurance of safety, Siglec-15 inhibition can be widely exploited and has clinical utility.
(3) Siglec-15 is similar to FGL1-LAG-3 and conforms to the three normalization principles mentioned above. It is a potential therapeutic target for normalization cancer immunotherapy and is in line with the future trend of immunotherapy.
Perspective
Immunosuppression in the TME has always been a hindrance in the treatment of cancer. The normalization cancer immunotherapy proposed by Professor Chen focuses on blocking various immunosuppressive pathways in TME, correcting local immunodeficiency, and normalizing anti-tumor immunity. However, we must remember that immunosuppression is a complex network, and that anti-PD-1/PD-L1 therapy only has good effects in a subset of patients, suggesting that there are other immunosuppressive molecules in the tumor immune response. The discovery of Siglec-15 is a solid indication of this. The proposition of normalization cancer immunotherapy and the development of TCAA have also inspired researchers to discover more key inhibitory molecules in tumor immune escape, which should lead to the development of treatments against these molecules to compensate for the limitations of targeting a single immunosuppressive molecule.
However, immunotherapy still has some unresolved issues. They include the following:
(1) The TME is highly heterogeneous, and tumor-induced immune deficiency is also heterogeneous19. The major immunodeficiency differs in from patient-to-patient and between different regions in a single tumor lesion. These heterogeneities may affect the therapeutic effect, making the treatment more complicated. We should consider the characteristics of each patient, or convert different TMEs into similar patterns and implement a specific treatment to achieve precise treatment.
(2) At present, in addition to PD-1/PD-L1 and Siglec-15, some other immunosuppressive checkpoints (Tim3, TIGIT, CD73, etc.)20,21 have been found. Some of these studies were retrospective and few studies have addressed whether there is any correlation between them and how they may interact.
(3) Among many of the immunosuppressive pathways that have been discovered so far, which is the master switch that dominates the TME? Previous studies have suggested that PD-1/PD-L1 is the master switch of local tumor immunity. It is not necessary to correct all defects in the TME. Blocking the PD-1/PD-L1 pathway alone may trigger the remodeling of local tumor immunity and restore anti-tumor effects2. With the discovery of additional and important immunosuppressive pathways, this view may need to be changed, and there may be a more powerful and comprehensive "master switch" than the PD-1/PD-L1 pathway, which needs further investigation.
(4) This study has repeatedly confirmed the strong immunosuppressive effect of Siglec-15 in animal models. The actual role of this molecule in human tumors, as well as the actual anti-tumor effect of blocking Siglec-15, has yet to be verified by more preclinical researches and clinical trials. Professor Chen has remarked that Siglec-15 is a major immune suppressor in PD-L1 negative tumors, which are resistant to currently approved anti-PD-1/PD-L1 therapies. The hope is that Siglec-15 will be the first in a series of novel targets for immunomedicines to help patients who do not respond to current immunotherapy medicines. Siglec-15 knock-out mice do not develop autoimmune or other diseases, suggesting that Siglec-15 inhibition may not be associated with adverse effects on normal cells. NC318, a first-in-class immunomedicine against Siglec-15, blocked the negative effects of Siglec-15. A first-in-human phase I/II clinical trial is ongoing for NC318 (NCT03665285) and began recruiting patients at the end of 2018, with indications for a variety of solid tumors, including lung cancer, uterine cancer, and head and neck cancer. The phase II is anticipated to be complete in May 2021.
Normalization cancer immunotherapy is the future trend. Maintaining homeostasis and balance of the body, not activating or inhibiting a certain function excessively, is consistent with the natural laws of the human body. I believe that after further exploration and solving the problems that have been considered above, immunotherapy under the guidance of immune normalization principles will be advantageous for the eradication of human cancers.
Conflict of interest statement
No potential conflicts of interest are disclosed.
- Received April 16, 2019.
- Accepted May 10, 2019.
- Copyright: © 2019, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.