Abstract
Objective: Long non-coding RNAs (lncRNAs) are involved in numerous biological processes in lung cancer cells. In our previous studies, we identified a lncRNA, ENST00000439577, which is highly expressed in lung carcinomas, and termed it lung cancer progression-associated transcript 1 (LCPAT1). To characterize the role of LCPAT1 in lung cancer, we conducted the current study.
Methods: Expression of LCPAT1 and autophagy-associated markers in tumor tissues and lung cancer cell lines was determined by real-time quantitative polymerase chain reaction (qPCR). Hematoxylin and eosin (HE) staining, qPCR, Western blot, and immunohistochemistry were performed to evaluate xenografted tumor tissues. Autophagy induced by rapamycin was detected by Western blot and immunofluorescence in lung cancer cell lines.
Results: Expression of LCPAT1 and microtubule-associated protein 1 light chain 3 beta (LC3B) was positively correlated in lung cancer. Knockdown of LCPAT1 inhibited tumor growth and suppressed cell autophagy in vivo. Moreover, LCPAT1 knockdown in lung cancer cell lines resulted in decreased autophagy-associated gene expression and alleviated the cell autophagy induced by rapamycin.
Conclusions: We speculate that LCPAT1 plays a crucial role in regulating autophagy in lung cancer.
keywords
Introduction
Lung cancer is one of the leading causes of cancer deaths worldwide1. The most common type of lung cancer is non-small cell lung cancer (NSCLC) and its 5-year survival rate is < 15% 2. Although extensive research has been carried out, the pathological process of lung cancer remains unclear. For the past few years, long non-coding RNAs (lncRNAs) have garnered increasing attention in lung cancer research, both as modulators of pathogenesis and as potential therapeutic targets3-5.
LncRNAs are a class of non-protein coding transcripts that are 200 nucleotides or longer in length6. Though they do not code for proteins, lncRNAs control gene expression by functioning as guides, scaffolds, and decoys of many biomolecules. Also, transcription of lncRNAs is exquisitely regulated and the expression of lncRNAs reflects different developmental stages or responds to diverse extracellular signals7. LncRNAs are now recognized as novel regulators in tumor development and progression. It is well-known that the lncRNAs H198, HOTAIR9, MEG310, MALAT111, and ANRIL3 are dysregulated in cancer. In our previous study, we reported that expression of LOC146880 and ENST00000439577 was elevated in lung cancer5.
ENST00000439577 is a 311-bp transcript and its gene is located on chromosome 1 (chr1: 17, 406, 760-17, 407, 382). We previously reported that ENST00000439577 expression was associated with metastasis status and disease stage in lung cancer patients. Moreover, high expression of ENST00000439577 was associated with poor overall survival of lung cancer patients5. Thus, we termed ENST00000439577 lung cancer progression-associated transcript 1 (LCPAT1), suggesting that LCPAT1 may act as an oncogene in lung cancer.
LncRNAs participate in tumorigenesis through multiple pathways, including autophagy11-15. Autophagy is an intracellular bulk degradation process through which proteins, lipids, and organelles are delivered to lysosomes for degradation16. In this process, autophagy related 5 (ATG5), autophagy related 14 (ATG14), BECLIN1, and other autophagy-related genes play an important role. Sequestosome 1 (p62) is incorporated into mature autophagosomes and degraded during autophagy. Microtubule-associated protein 1 light chain 3 beta (LC3B) levels increase and LC3B-encoded LC3-I is converted to LC3-II which accumulates and adsorbs on the autophagosome membrane. The degradation of p62 and increase of LC3-II are commonly used as markers of autophagy17.
Autophagy acts as a double-edged sword in cell survival and cell death18. In the process of tumor development, autophagy suppresses chronic inflammatory reactions to prevent tumorigenesis, and enable tumor survival by recycling nutrients19. Genetic deletion of an autophagy protein, ATG5, accelerates the initiation of KRas (G12D)-driven lung tumors in mice, but impairs the progression of lung cancer, suggesting that autophagy may prevent oncogenesis, but promote tumor growth20.
Several lncRNAs targeting autophagy have been described. The overexpression of the lncRNA BANCR results in an increase in the ratio of LC3II-to-LC3I, which indicates the formation of an autophagosome21. Our previous study revealed that the lncRNA LOC146880 participates in PM2.5 exposure-induced autophagy in lung cancer4. The purpose of the current study was to determine the role of LCPAT1 in regulating autophagy in vivo and in vitro. We found that LCPAT1 was positively correlated with LC3B in lung cancer. In vitro and in vivo evidence demonstrated that LCPAT1 knockdown inhibits activation of autophagy in lung cancer. These findings indicate the pivotal role of lncRNA LCPAT1 in lung cancer and the possible mechanisms involved with autophagy.
Materials and methods
Patients
Tumor samples and matched adjacent normal tissues were collected with informed consent from lung cancer patients during surgeries performed between May 2006 and July 2011 at the Tianjin Medical University Cancer Institute and Hospital (TMUCIH). RNA extraction and analysis were performed as previously described4,5,22. The current study was approved by the medical ethical review committees at TMUCIH and Shanghai Jiao Tong University School of Medicine.
Cell culture
HEK-293T and lung cancer cell lines, A549 and H1975, were obtained from the American Type Culture Collection (ATCC). All cell lines were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) and 100 U penicillin-streptomycin under 5% CO2 at 37 °C.
Lentiviral small hairpin (sh)RNA
Plasmids for shRNA targeting LCPAT1 (sense, 5′-CAATGTTGTTGTTTATTTA-3′ and antisense, 5′-TAAATAAACAACAACATTG-3′) and scrambled (sense, 5′-TTCTCCGAACGTGTCACGT-3′ and antisense, 5′-ACGTGACACGTTCGGAGAA-3′) were obtained from Shanghai Integrated Biotech Solution Company and the interfering vector used to generate shRNA was pLKD-CMV-G & PR-U6-shRNA. Lentiviral vector DNA and package vectors were transfected into HEK-293T cells by Lipofectamine® 2000 transfection reagent (Invitrogen). At 48 and 72 h after transfection, lentivirus supernatants were harvested and used to infect H1975 cells. Stable shRNA cell lines were generated following selection with 1 μg/mL puromycin. The efficiency of gene knockdown was detected by real-time quantitative polymerase chain reaction (qPCR).
Animals
BALB/c mice, 4–6 weeks old, were obtained from Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China) and raised in a specific-pathogen-free grade environment. Three female and three male mice were used for a control experiment. H1975 cells transfected with scrambled shRNAs or LCPAT1-shRNA were suspended in phosphate-buffered saline with Matrigel matrix basement membrane extract (Bedford, MA, USA) in a 1:1 ratio. Then, the cell solutions (5 × 106 cells/100 μL/mouse) were injected subcutaneously in the left and right hind flanks of mice. Tumor growth was evaluated by measuring tumor diameters every 2 days with a vernier caliper, and tumor volume was calculated using the following formula: V = 1/2×a×b2, where V = tumor volume, a = the larger perpendicular diameter, and b = the smaller perpendicular diameter. The animals were sacrificed on day 21 after implantation and tumors were collected for analysis. All applicable guidelines of the Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine were followed.
Hematoxylin and eosin (HE) staining
Xenografted tumor tissues were fixed with 4% paraformaldehyde (BOSTER, Wuhan, China), embedded in paraffin, sectioned, and visualized at 100× and 200× magnification after HE staining.
Immunohistochemical (IHC) staining
For lung cancer patients’ tissue, the sections (5 µm) from the tissue microarray blocks were labelled with antibodies. The LC3B antibody was diluted 1:2000. An HRP- conjugated secondary antibody (Beijing Sequoia Jinqiao Biological Technology Co., Ltd.) was used. The specific target was visualized with a 3, 3’-diaminobenzidine (DAB) detection kit (Beijing Sequoia Jinqiao Biological Technology Co., Ltd.) and counterstained with hematoxylin. Microphotographs from each arrayed tissue were taken with a fixed exposure time and color balance to ensure consistency. LC3B production was quantified using ImagePro Plus9.1 (Media Cybernetic, Silver Spring, MD).
For animal tumor tissue, paraffin sections were first stained with anti-ATG5 (#ab108327, 1:50; Abcam, Cambridge, MA, USA) and anti- LC3B (#ab48394, 1:50; Abcam). Horseradish peroxidase (DAKO, Glostrup, Denmark) was then used as a secondary antibody. When an antigen-antibody-antibody complex was formed, a substrate of the peroxidase, diaminobenzidine, was added as chromogen. The staining was performed according to the manufacturer’s instructions. The pictures were taken at a magnification of 400 × and analyzed using Image Pro-plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA). Five different pictures were measured for each sample.
Small interfering RNA (siRNA) transfectionss
Control and LCPAT1-specific small interfering RNA were obtained from Genepharma (Shanghai, China). Three specific siRNAs for LCPAT1 were designed and mixed for transfection. The sequences of LCPAT1 siRNAs are listed in Table 1. Cells (3 × 105) were seeded into six-well plates and transfected with siRNAs after overnight incubation at 37 °C. The transfection was performed with Lipofectamine® 2000 transfection reagent (Invitrogen) according to the manufacturer’s protocol .
Sequences of siRNAs
Real-time quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from cells and tissues using TRIzol, and cDNA was synthesized using a FastQuant RT Kit with gDNase (TIANGEN, Beijing, China). The primers used for analyzing the expression of LCPAT1, BECLIN1, LC3B, p62, ATG3, ATG5, ATG7, ATG12, ATG14, and β-ACTIN were synthesized by Sangon Biotech (Shanghai, China) (Table 2). Quantitative RT-PCR was performed in the ABI Prism 7500 system (Applied Biosystems, Foster City, CA, USA) using the SYBR Green Supermix (Applied Biosystems). Relative gene expression was analyzed according to the formula: 2−∆∆CT.
Sequences of PCR primers
Western blot analysis
Tissue samples and cultured cells were lysed with RIPA lysis buffer containing protease inhibitor. SDS-PAGE was used to detect the proteins of interest. Proteins were resolved on SDS gels and were electro-transferred to polyvinylidene fluoride membranes (Millipore, Boston, MA, USA). Then, the membranes were blocked with 5% non-fat milk and incubated overnight at 4°C with primary antibodies, including anti-LC3B (#ab48394, 1:500; Abcam), anti-p62 (#ab56416, 1:500; Abcam), anti-ATG5 (#ab108327, 1:500; Abcam), anti-BECLIN1 (#ab55877, 1:500; Abcam), and anti-β-ACTIN (#A8481, 1:4000; Sigma-Aldrich). After washing, the membranes were incubated with a secondary antibody and imaged with Odyssey SA (Gene Company Limited, Hong Kong, China).
Immunofluorescence assay
Cultured cells were grown on glass coverslips overnight to detect the LC3B puncta. Cells fixed with 4% paraformaldehyde (BOSTER) were treated with 0.2% Triton X-100 and blocked with 2% albumin from bovine serum (BSA). Then, cells were incubated with a primary anti- LC3B antibody (#ab48394, 1:200; Abcam) at 4 °C overnight. After the incubation of Alexa Fluor 568-conjugated goat anti-rabbit IgG (#ab175694, 1:1000; Abcam), cells were stained with 0.2 mg/mL DAPI and imaged with a confocal microscope (Zeiss LSM710, Carl Zeiss, Dresden, Germany). The LC3B puncta were measured using Image Pro-plus 6.0 software (Media Cybernetics). Five different pictures were measured for each group.
Statistical analysis
All data were analyzed using the SPSS software package, version 19.0 (SPSS, Chicago, IL, USA) and graphed by GraphPad Prism 5 (La Jolla, CA, USA). The results were presented as mean ± standard error based on three independent experiments. Statistical analysis was performed using correlation analysis and t test. P value < 0.05 was defined as statistically significant.
Results
Correlation between LCPAT1 and LC3B in lung cancer
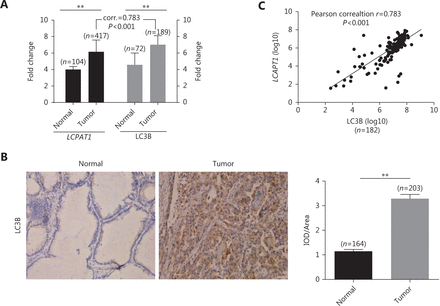
In lung cancer patients, tumor tissues showed higher LCPAT1 and LC3B expression versus normal tissues at the gene expression level (Figure 1A). Also, IHC staining showed that the cytoplasmicLC3B protein was highly expressed in lung tumor tissues (Figure 1B). To investigate the relationship between LCPAT1 and autophagy, we analyzed the correlation of LCPAT1 and LC3B expression in tumor tissues. Our results showed that the correlation coefficient between these genes was high, r = 0.783 (Figure 1C), suggesting that LCPAT1 may play a role in autophagy in lung cancer.
Correlation between LCPAT1 and LC3B expression in lung cancer. (A) Gene expression of LCPAT1 and LC3B in tumor tissues and matched adjacent normal tissues of lung cancer patients. Data are presented in mean ± SEM; **P < 0.01. (B) Representative immunohistochemical staining images (400 ×) of cytoplasmic LC3B and its density (IOD/Aera) in normal and lung cancer tissues. (C) Correlation between LCPAT1 and LC3B expression in tumor tissues of lung cancer patients.
Suppression of tumor growth in vivo by LCPAT1 knockdown
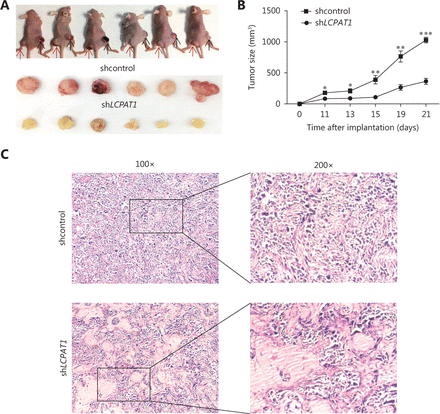
We evaluated the involvement of LCPAT1 in tumor growth in vivo. H1975 cells transfected with scrambled shRNAs or LCPAT1-shRNA were injected subcutaneously into nude mice. Tumor growth was recorded and analyzed after the injection. We observed smaller tumor sizes in the right hind flanks of animals that were injected with LCPAT1-shRNA (Figure 2A). We also found that tumor growth was slower in the LCPAT1-shRNA tumors compared with that of the controls (Figure 2B). HE tissue staining revealed that tumors with LCPAT1-shRNA possessed less malignant morphology compared to the controls (Figure 2C). These findings suggest that LCPAT1 may promote tumor growth.
LCPAT1 knockdown xenograft models. (A) Upper: LCPAT1-shRNA (sh LCPAT1)- and control shRNA (shcontrol)-transfected H1975 cells were implanted in flanks of mice (n = 6). The red arrow indicates the tumors implanted with shLCPAT1-transfected H1975 cells on the left side, and the black arrow indicates the tumors implanted with shcontrol-transfected H1975 cells on the right side. Lower: tumors expressing shLCPAT1 or shcontrol. (B) Tumor growth curve. Data are presented as mean ± SEM; *P < 0.05, ** P < 0.01, *** P < 0.001. (C) H & E staining of xenografted tumor tissues of sh LCPAT1- and shcontrol-transfected H1975 cells (Left: 100 ×. Right: 200 ×).
Suppression of autophagy by LCPAT1 knockdown in vivo
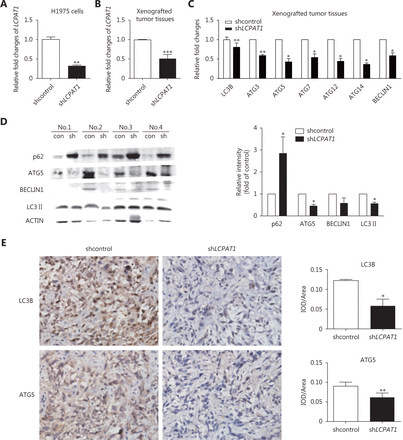
As shown in Figure 3A and 3B, LCPAT1 expression was reduced by nearly 60% in stable LCPAT1 knockdown H1975 cells and 50% in LCPAT1 knockdown tumors. We found that the expression of autophagy-associated genes was significantly reduced in LCPAT1-shRNA tumors compared to that in the controls, with an approximately 50% drop in LC3B, 59% drop in ATG3, 42% drop in ATG5, 54% drop in ATG7, 44% drop in ATG12, 36% drop in ATG14, and 59% drop in BECLIN1 (Figure 3C). We further validated our findings using Western blot. We observed that LCPAT1 knockdown significantly suppressed the expression of ATG5 (approximately 48% reduction) and LC3-II (approximately 57% reduction) and stimulated the expression of p62 (approximate 2.5-fold increase; Figure 3D). Additionally, the immunohistochemical analysis of LC3B and ATG5 showed a decreasing trend in shLCPAT1 xenografted tumor tissues (Figure 3E). Taken together, autophagy appeared to be suppressed after LCPAT1 knockdown in vivo.
Autophagy regulation by LCPAT1 in vivo. (A) qPCR analysis of the expression of LCPAT1 in stable-transfected H1975 cell line. Data are presented as mean ± SEM; **P < 0.01. (B) qPCR analysis of the expression of LCPAT1 in xenografted tumor tissues. Data are presented in mean ± SEM; ***P < 0.001 ( n = 6). (C) qPCR analysis of the expression of LC3B, ATG3, ATG5, ATG12, ATG14, and BECLIN1 in shLCPAT1 and shcontrol xenografted tumor tissues (n = 6). Data are presented in mean ± SEM; *P < 0.05, ** P < 0.01, *** P < 0.001. (D) Representative image and quantification of Western blot assays for p62, ATG5, BECLIN1, and LC3B expression in sh LCPAT1 and shcontrol xenografted tumor tissues (n = 4). Nos.1-4 represent the number of mice. Data are presented in mean ± SEM; *P < 0.05. (E) Representative image (400 ×) and quantification of immunohistochemical assays for LC3B and ATG5 expression in sh LCPAT1 and shcontrol xenografted tumor tissues using Image Pro-plus 6.0 software (n = 4). Data are presented in mean ± SEM; *P < 0.05, ** P < 0.01.
Suppression of autophagy by LCPAT1 knockdown in vitro
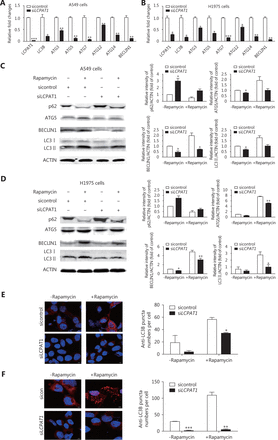
Based on the above in vivo findings, we further investigated the role of LCPAT1 in lung cancer cells. After detecting the expression of LCPAT1 in the normal bronchial epithelial cell line HBE and lung cancer cell lines H520, H1299, A549, and H1975, we found that LCPAT1 expression was higher in lung cancer cell lines than in HBE, especially in A549 and H1975 (Figure S1). It was observed that when LCPAT1-siRNA was transfected into A549 and H1975 cells (Figure 4A and 4B), mRNA expression of autophagy-associated genes was markedly decreased. For example, in H1975 cell lines, LC3B, ATG5, ATG7, ATG14, and BECLIN1 were decreased by 60%–80%, and ATG3 and ATG12 were reduced by 40%. In addition, upon treating cells with rapamycin, an autophagy inducer, we observed the accumulation of ATG5, BECLIN1, and LC3-II, and the degradation of p62. However, this effect was inhibited when LCPAT1 was silenced (Figure 4C and 4D). Furthermore, the assembly of rapamycin-induced LC3B puncta was inhibited in LCPAT1 knockdown cells based on immunofluorescence assays (Figure 4E and 4F). These experiments showed that LCPAT1 regulated cell autophagy in vitro.
Expression of LCPAT1 in normal bronchial epithelial cell line and lung cancer cell lines. The expression of LCPAT1 in the normal bronchial epithelial cell line HBE and lung cancer cell lines H520, H1299, A549, and H1975 was detected by qPCR.
Autophagy regulation by LCPAT1 in vitro. (A, B) mRNA expression of autophagy-related genes in A549 cells (A) and H1975 cells (B) transfected with LCPAT1 siRNA (siLCPAT1) and scrambled siRNA (sicontrol). Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001. (C, D) Representative images and quantification of Western blot assays for p62, ATG5, BECLIN1, and LC3B expression in siLCPAT1- and sicontrol-transfected A549 cells (C) and H1975 cells (D), after exposure to 100 μM rapamycin for 48 h. Data are presented in mean ± SEM; *P < 0.05, **P < 0.01. (E, F) Representative images and quantification of LC3 dots in siLCPAT1- and sicontrol-transfected A549 cells (E) and H1975 cells (F) after treatment with 100 μM rapamycin for 48 h. The LC3B puncta were quantified by Image Pro-plus 6.0 software. Data are presented in mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Following our previous work on lncRNA LCPAT1, we further analyzed its relationship with autophagy in lung cancer in the current study. We found a strong positive correlation between LCPAT1 and LC3B. Since high expression of LC3B indicates increased autophagy17, we speculate that LCPAT1 may promote autophagy in lung cancer. We investigated the role of LCPAT1 in autophagy by knocking down LCPAT1 expression in tumor cells and assessing its impact on autophagy in vitro and in vivo. Our results demonstrated that LCPAT1 could increase autophagy in lung cancer.
More and more lncRNAs are being found to be involved in tumorigenesis. In lung cancer, HOTAIR is up-regulated and the up-regulation promotes tumor cell invasion and metastasis23. In contrast to HOTAIR, MEG3 is significantly down-regulated in lung cancer; increasing its expression in lung cancer cells could inhibit cell proliferation and induce apoptosis24. Considering that a large number of lncRNAs are known and only a few are recognized for their involvement in lung cancer, we previously performed an expression microarray analysis to identify dysregulated lncRNAs in lung cancer5. A methylation microarray chip was also used to determine the status of DNA methylation in the same samples. Among 8500 differentially-expressed lncRNAs, expression of LOC146880 and LCPAT1 was found to be elevated in tumors and the expression was negatively correlated with DNA methylation in the promoter. We further noticed that cell proliferation, invasion, and migration were inhibited when lowering the expression of LOC146880 or LCPAT1 in lung cancer cells5. In the present study, our animal experiments further confirmed that suppressing LCPAT1 expression by shRNA could inhibit tumor growth in a xenograft model.
Autophagy plays a complicated role in tumorigenesis. Autophagy can suppress cancer initiation during the early stage of tumorigenesis. Karantza-Wadsworth et al.25 reported that a defect in autophagy led to DNA damage, genomic instability, and further promoted the activation of proto-oncogenes and resulted in tumorigenesis. In contrast, autophagy promotes the growth of established cancer25. In malignant glioma, autophagy can be activated by hypoxia, thereby promoting tumor cell growth through the HIF-1α/AMPK signaling pathway26. Our finding of high expression of LC3B in lung cancer supports the notion that autophagy promotes cancer progression. We also found that LCPAT1 could increase autophagy in lung cancer, suggesting that LCPAT1 may act as an oncogene by promoting autophagy in lung cancer.
The molecular mechanisms underlying how LCPAT1 regulates autophagy are unclear. LncRNAs regulate the expression of genes through various mechanisms, including interacting with DNA, mRNAs, microRNAs, or proteins. In breast cancer, linc-ROR has been shown to suppress gemcitabine-induced autophagy by silencing miR-34a27. Through binding to EZH2, lncRNA H19 down-regulates DIRAS3 expression, consequently inhibiting autophagy in cardiomyocytes28. Wu et al.29 has reported that the lncRNA HNF1A-AS1 promotes autophagy in hepatocellular carcinoma by composing a competitive endogenous RNAs system with miR-30b, which targeted ATG529. We used starBase (http://starbase.sysu.edu.cn/)30 and lncRNASNP (http://bioinfo.life.hust.edu.cn/lncRNASNP/)31 databases to predict whether there were miRNA binding sites for LCPAT1 and autophagy-related genes. The results showed that mir-186-5p binds to LCPAT1, ATG7, and ATG12. In addition, mir-206 can be combined with LCPAT1 and ATG14 at the same time, and mir-218-5p can be combined with LCPAT1, ATG12, and ATG14 at the same time. Studies have shown that mir-186-5p, mir-206, and mir-218-5p are all tumor suppressors 32-34. We suspect that LCPAT1 can compete with autophagy-related genes for specific anti-cancer miRNAs, thereby elevating autophagy gene levels and exerting tumorigenic effects. The current study was an initial investigation into the role played by LCPAT1 in lung cancer. Further studies are warranted to explore the mechanisms of LCPAT1 in regulating autophagy in lung cancer.
In conclusion, we confirm that LCPAT1 is a tumor-promoting lncRNA in lung cancer. We also found that LCPAT1 could increase autophagy both in vitro and in vivo in lung cancer.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (Grant No. 81401046 and No. 21777099), Shanghai Jiao Tong University Interdisciplinary Research Key Grant (Grant No. YG2015ZD01) and Shanghai Jiao Tong University “New Young Teachers Startup Plan” .
Footnotes
↵* These authors have contributed equally to this work.
Conflict of interest statement No potential conflicts of interest are disclosed.
- Received October 23, 2018.
- Accepted May 31, 2018.
- Copyright: © 2018, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.