Abstract
Mesenchymal stromal cells (MSCs) are adult multipotent stem cells residing as pericytes in various tissues and organs where they can differentiate into specialized cells to replace dying cells and damaged tissues. These cells are commonly found at injury sites and in tumors that are known to behave like “wounds that do not heal.” In this article, we discuss the mechanisms of MSCs in migrating, homing, and repairing injured tissues. We also review a number of reports showing that tumor microenvironment triggers plasticity mechanisms in MSCs to induce malignant neoplastic tissue formation, maintenance, and chemoresistance, as well as tumor growth. The antitumor properties and therapeutic potential of MSCs are also discussed.
keywords
- Mesenchymal stromal cells
- migration
- homing
- tumor modulation
- signaling pathways
- chemoresistance
- systemic circulation
Introduction
Mesenchymal stromal cells (MSCs) are immature, adherent stromal cells residing in various tissues and organs, including bone marrow (BM-MSCs), adipose tissue (AT-MSCs), umbilical cord blood, and placenta. The presence of circulating MSCs in the peripheral blood is still debated1,2. MSCs divide into daughter cells that share the same properties of their mother cell (self-renewal) or differentiate into specialized cells to replace dying cells and repair damaged tissues (multilineage differentiation). Notably, these adult stem cells can differentiate into various cell types of the mesodermal lineage, including chondrocytes, osteoblasts, adipocytes, endothelial cells, and myocytes3; moreover, non-mesodermal differentiation into neural, liver, pancreatic, and gastric cells has been reported in vitro, but this phenomenon occurring in vivo has not been proven4–7. MSCs express membrane CD90, CD73, and CD105, and are negative for CD45, CD34, CD31, CD14, CD19, and HLA-DR3,8.
Emerging data suggest that MSCs can promote tumorigenic processes, including malignant transformation, establishment and maintenance of cancer cells, promotion of angiogenesis and neovascularization-sustaining neoplastic tissues, metastasis formation, and chemoresistance to anticancer drugs9–11. MSCs have the capability to contribute to the formation of cancer stem cell niche and support stemness9–11. In this article, we provide an overview on the MSC properties that drive their tissue repair capability, such as migration, adhesion, differentiation, growth factor production, and immune regulation. Then, we discuss how the same features may boost tumor development and favor chemoresistance mediated by the tumor microenvironment.
MSCs, regenerative medicine, and cell therapy
Therapeutic potential of embryonic and adult stem cells
Tissue or organ transplantation is still associated with various issues, including inadequate donor availability, compatibility between donors and recipients, and risk of developing graft-related complications. Stem cell transplantation has emerged as a promising strategy to replace or improve organ transplantation12,13. The premise is that stem cells, once administered to the recipient with organ failure, migrate to the damaged sites and differentiate into the specific affected cell types to restore/replace damaged tissues and rescue organ functions. Stem cells can be classified as embryonic stem cells, which give rise to all tissue types, and adult stem cells, which are involved in the tissue homeostasis by replacing senescent or damaged cells based on their differentiation potency and developmental hierarchy. The high proliferation rate and pluripotency of embryonic stem cells, that is, the ability to differentiate into virtually all cell types of the three germinal layers (ectoderm, mesoderm, and endoderm), would make them the optimal model for tissue engineering, regardless of their potential immunogenicity. However, their therapeutic use is entangled with critical ethical issues and uncontrolled proliferation, leading to teratoma formation in vivo; moreover, the latter issue remains unsolved even with the use of alternative technologies aimed at achieving embryonic stem cell-like cells, such as induced pluripotent stem cells14,15. Consequently, adult stem cells have rapidly become the main tool in regenerative medicine and tissue engineering because of their high proliferative and differentiation properties, easy collection, and weak immunogenicity16–20.
MSC therapeutic potential
MSC use is a first attempt in adult stem cells-based therapy. A body of evidence from in vivo and in vitro studies shows that MSCs possess regenerative potential associated to their adhesion, migration, proliferation, differentiation, and immunosuppression properties21–24. This adult stem cell type is highly used in preclinical studies and phase 2 and 3 clinical trials aimed at mitigating graft-versus-host disease (GvHD) and at regenerating damaged tissues in many diseases and conditions that are thought to originate from deleterious damages to tissues16,25. Examples include the attempts to regenerate bone, heart, muscle, and nervous tissues following tissue injury from inflammation- and oxidative stress-associated pathogenic processes26,27. Tissue repair and attenuation of chronic or acute inflammation were observed after local or systemic infusion of MSC in patients16,25. However, the real clinical impact of this cell therapy approach remains unknown and requires further multicenter studies based on standardized methods to assess safety and efficacy.
MSCs have been well characterized with respect to their ability to produce a range of growth factors and cytokines, which inspired the designation of these cells as an “injury drugstore”28. Notably, MSC secretome screening revealed numerous growth factors that potentially contribute to tissue repair, such as (i) vascular endothelial growth factor (VEGF), which has angiogenic abilities and triggers endothelial differentiation in MSCs through VEGFR-2/Sox1829 and Rho/myocardin-related transcription factor-dependent mechanisms, thereby promoting blood vessel repair30,31; (ii) hepatocyte growth factor (HGF) that may play a role in MSC regenerative effects on the liver, as it promotes the differentiation and proliferation of hepatic-like cells and induces MSC-associated cytoprotective effects on hepatocytes in vivo32–34; (iii) transforming growth factor-beta (TGF-β), whose involvement was reported in MSC-mediated heart repair, where it stimulated the differentiation of cardiomyocytes and promoted angiogenesis35; (iv) angiopoietin-1, another pro-angiogenic factor involved in MSC-mediated improvement of cardiac function (36) and skin damage37; (v) epidermal growth factor (EGF) that mediates MSC-associated protection of podocytes from high glucose-induced apoptosis38; (vi) platelet-derived growth factor (PDGF), whose release by MSCs was reported to play a role in cardiac healing after myocardial injury by exerting a pro-migratory effect on resident cardiac stem cells39; (vii) granulocyte-colony stimulating factor (G-CSF), whose release by MSCs is triggered by co-cultures with counter-inflammatory or tissue repair macrophages, enhanced by the MSC cartilage-forming capacity 40; and (viii) fibroblast growth factor (FGF), and cytoprotective factors that partly account for the therapeutic effects of MSCs in lung diseases41. Numerous other soluble factors are released by MSCs and contribute to the properties of these cells, including stem cell factor, MCP-3, CXCL8, CXCL9, CXCL16, CCL20, CCL25, IL-6, and IL-1242–45.
MSC properties contributing to tissue repair ability
Migration and homing
Determinant factors of cell therapeutic potential include migration, homing, and survival when administered through a specific route. Labeling and tracking of MSCs have been employed to understand the MSC distribution in the body following local or systemic injection. Reported labeling approaches include intracellular magnetic contrast materials, radioactive compound, and fluorescent dyes46–48; and expression systems, such as luciferase, green fluorescent protein (GFP), and Alu sequences48–52. Studies addressing the final location of MSCs after systemic infusion in disease-free laboratory animals revealed that injected MSCs could localize in diverse sites of organs, such as the lung, liver, and spleen53,54. Interestingly, comparable studies in animals with damaged organs revealed a tropism of injected MSCs for damaged sites, particularly following administration at neighboring areas. For instance, Barbash and colleagues54 reported that 99mTc-exametazime-labeled MSCs infused in the left ventricle cavity, instead of intravenously, resulted in a drastically reduced lung uptake and increased infarcted myocardium uptake in a rat model. Comparably, after palatine tonsil MSCs (T-MSCs) were intravenously administered to carbon tetrachloride-induced mouse model of liver fibrosis, the T-MSCs were only found in the liver55. Intravenously injected MSCs migrated, distributed to the colon, and effectively mitigated disease severity indicators in a rat model of ulcerative colitis via an anti-inflammatory effect partly mediated by G-CSF56. Moreover, nasal mucosa ecto-mesenchymal stromal cells injected in the tail vein migrated to the inflammation sites and suppressed eosinophils and sneezing in a mouse model of allergic rhinitis via downregulation of Th-2 cell secretory activity, that is, decreases in IgE, IL-4, IL-5, and IL-10 secretions; and upregulation of Th-1 cell secretion, including the release of IgG2 and IFN-γ57. Interestingly, MSC delivery into the arterial system via injection into the aortic arch or tail vein supports the “first-pass” cell delivery hypothesis. Indeed, MSCs showed significant entrapment in the lungs when delivered intravenously into the tail vein. However, when delivered intra-arterially through the aortic arch, the cells were highly and evenly distributed in the entire animal45. MSCs were reported to home at the sites of ischemia, hypoxia, inflammation, and other injuries54,55,58. Overall, such sites have high concentrations of pro-inflammatory cytokines, chemokines, and soluble factors that may attract MSCs and favor their homing21,22.
The migration and homing of infused MSCs to damaged tissues are important parameters to consider for clinical purposes. Failure or poor results following attempts of MSC-based therapy observed in a significant number of patients raised at least three concerns. First is their overall viability following infusion via the route used (local versus systemic43); moreover, despite clinical evidence and reports from experimental models supporting the assumption that MSC homing may be governed by damaged tissues, MSCs can still be found in unwanted sites, thereby raising safety concerns for the long-term effects of MSC-based therapy59,60, particularly in pediatric patients61–63. Second is the engraftment degree of MSCs in targeted tissues, and the third, as a consequence, is the fate of these cells if improperly engrafted. Improving the MSC viability and therapeutic potency is currently a challenge. A remarkable example is provided by MSC-based therapy in lung diseases, where MSC grafting and homing to affected tissues are successful. This therapeutic approach has been held back by the difficulty of engrafted MSCs to survive more than one week after-transplantation in hostile microenvironments41. Similar observations have been reported in other injuries, including renal ischemia/reperfusion injury, where hypoxia preconditioning of MSCs appeared as a possible solution64. Hypoxia induces the secretion of anti-inflammatory, antiapoptotic, and anti-fibrotic factors, as well as the expression of cytoprotective genes, thereby enhancing the therapeutic potential and survival duration of the engrafted MSCs41,64. Recently, a study in human umbilical cord MSCs suggested the three-dimensional spheroid culture of these stem cells as a strategy to promote cell yield and stemness maintenance65.
Moreover, functional differences were reported in MSCs from human dental pulp and periodontal ligaments66, indicating that MSCs from topographically related tissues do not necessarily share identical properties, thereby emphasizing the need for comparing the multipotency, immunosuppression properties, response to pro-inflammatory cytokines, and eventually the secretome of MSCs from diverse sources before clinical use. Reports in various human studies21,22 and animal models23,24 corroborated these observations.
Differentiation and transdifferentiation
The differentiation ability of MSCs accounts for their positive effects in diseases wherein pathogenic processes include severe tissue damage, such as in cardiac lesions. For instance, a promising observation in MSC-mediated therapy was the finding that injecting MSCs in an infarcted heart generates a new tissue made up of proliferating myocyte and vascular structures67. In a study with enhanced-GFP-labeled MSC, the regenerated cardiomyocytes, vascular smooth muscle, and endothelial cells were EGFP+, suggesting that the new heart tissue was mainly derived from MSC differentiation68. In a study where the injection of GFP-labeled AT-MSC in spinal cord of a canine model of acute spinal injury was associated with a functional recovery, GFP-positive cells at the injury site included cells positive for GFAP (astrocyte marker), Tuj-1, and NF160 (markers of immature post-mitotic neurons), suggesting that functional improvement was mediated by the differentiation of AT-MSCs into functional astrocytes and neurons69.
The plasticity of MSCs does not derive only from their ability to differentiate into other cell types (cell replacement), but includes fusion with resident cells, thereby resulting in the emergence of new cells capable of tissue-specific functions. This fusion is termed as lineage reprogramming or transdifferentiation. MSC transdifferentiation results in phenotypes that are highly related to resident cells70,71. Many studies report MSC ability to fuse with various cell types. Examples of such cell types include cardiomyocytes72,73, hepatocytes74,75, neurons, and corneal cells76,77. The increasing number of reports suggests that cell fusion is an alternate and a common and probably pivotal pathway in MSC plasticity.
Immune modulation
Besides the classic cell replacement (differentiation) and reprogramming (transdifferentiation) paradigms, immune modulatory properties contribute to the benefits of MSC therapy. MSCs may modulate immune responses using paracrine mechanisms and cell-cell interaction. Studies suggesting that the activating properties in MSCs are scarce include reports of the ability of MSC to activate allogeneic T-cells in mixed leukocyte reaction78, stimulate the activation and proliferation of resting T-cells in co-cultures79, the MSC behavior as conditional antigen presenting cells (APCs) in syngeneic immune responses80, the TLR-activated MSC ability to recruit and activate immune inflammatory cells81, and the secretion of pro-inflammatory cytokines and chemokines by MSCs42,44,45. However, the clinical implications of these observations are still unclear.
The immunosuppressive properties of MSCs are well documented. These effects may emerge from cell-cell interactions with both innate and adaptive immune system cells82–85, partly mediated by Toll-like receptor (TLR) pathways, as revealed by the immunosuppression effects of TLR4 activation (via mechanisms involving VCAM-1- and ICAM-1-mediated binding of immune cells) and TLR3 activation (via mechanisms that induce the formation of cable-like hyaluronic acid structures)86,87. The immunosuppressive abilities of MSC can be mediated by the release of soluble factors with anti-inflammatory effects, like indoleamine 2, 3-dioxygenase (IDO), inducible nitric oxide synthase (iNOS), prostaglandin E2 (PGE2), G-CSF, and TGF-β40,56,88–90. Such immunosuppressive effects account for the ability of MSC to inhibit inflammatory responses that are induced by the presence of transplanted tissues, thereby decreasing the probability of rejection91–95. MSCs prevents autoimmunity via CCL2-dependent recruitment of myeloid-derived suppressor cells, in a mouse model of experimental autoimmune uveitis96.
Clinical application of MSCs requires a relatively long-term ex vivo culture that results in cellular senescence and reduced therapeutic activity of transplanted cells97. Experimental evidence shows that the therapeutic potency of MSCs may be enhanced and even restored by improving the immunosuppressive properties of these cells. For instance, in a recent study, these properties were improved by using vitamin D receptor agonists as additives in a mouse model of sterile kidney inflammation98. This approach resulted in the suppression of Th17 and related inflammatory responses in the kidney. In another study, the MSC-activating neuropeptide, termed as substance P, potentiated the ability to secrete TGF-β1 in long-term culture MSCs, indicating a recovery of their immunosuppressive function97. Moreover, these cells recovered their ability to inactivate CD4+ cells in co-cultures (cell-cell contact). Adenoviral transduction of MSCs was proposed as a strategy for increasing the immunosuppressive properties of engrafted MSCs after cell transplantation66. Overall, because of their immune modulatory features, MSC are being tested to treat immune disorders, such as GvHD, rheumatoid arthritis, multiple sclerosis, type 1 diabetes, and inflammatory bowel disease, and to enhance transplant tolerance45.
MSCs and tumor microenvironment
MSC plasticity and tumorigenesis
A major role for MSCs in cancer development emerged from the fact that MSCs are commonly found in stromal niches of various tissues undergoing tumorigenesis, including bone marrow in hematological malignancies9–11, and in the affected ducts and lobules of breast cancer99–101. Moreover, MSC research insights raised concerns about the possibility of their role in all the developmental and maintenance steps of malignant tumors from initiation until the metastatic spread. Growing evidence supports the idea that MSCs may exploit the properties related to tissue repair to promote tumorigenesis and protect transforming cells from chemotherapy10,83,102–109. Therefore, events and mechanisms accounting for MSC-mediated tissue regeneration and repair, such as MSC activation, mobilization, migration, and homing to stromal microenvironment, differentiation and transdifferentiation, as well as the secretion of cytokines, growth factors, and other soluble factors modulating the local immune responses and improving stromal cell survival, may support the pro-oncogenic role of MSCs. Thus, MSCs may eventually facilitate cancer cell growth, partly by favoring the angiogenic and neovascularization processes that allow the survival of malignant neoplastic tissues107–109 and by modulating anticancer immunity and hijacking immune cells to favor tumor invasion and, subsequently, metastatic processes99,103–105,110. These findings have tempered the enthusiasm over the clinical application of stem cells and further raised safety concerns of the long-term use of these cells and which categories of patients may be suitable for MSC-based therapies.
Tumor stroma recapitulates damaged tissue microenvironment
MSC plastic properties generate pro-tumoral stroma
Tumor stroma mainly include immune, endothelial, and immune cells, such as lymphocytes, macrophages, neutrophils, and natural killer cells, as well as adipocytes, myofibroblasts, and carcinoma associated fibroblasts (CAFs)111. Among the most abundant tumor stroma components, CAFs considerably boost tumor growth, induce epithelial-mesenchymal transition, promote the acquisition of invasive phenotypes, and support angiogenesis112–115. Moreover, CAFs could induce epithelial-mesenchymal transition through paracrine TGF-β signaling115.
Besides, early in vitro studies revealed that, following long treatments with tumor cell-conditioned medium in vitro, MSCs can differentiate into CAFs116 via a TGFβ1/Smad3-dependent mechanism117,118. Growing evidence supports the ability of the MSC to differentiate into CAFs in vivo. For instance, MSCs differentiate into CAFs to promote metastatic tumors in advanced solid cancers118–124. Moreover, MSCs in tumors may display transdifferentiation, wherein fusion occurs with resident cells, such as malignant cells and other components of the tumor stroma, thereby resulting in the re-modeling of the tissue stroma of the affected organ into a pro-tumoral stroma103,107,125–127. Examples of MSC transdifferentiation include MSC fusion-induced reprogramming in lung cancer127, human melanoma107, breast cancer, and ovarian adenocarcinoma cells126. These reports further suggested that the plastic role of MSCs is a major pathogenic step because it drives the generation of a pro-tumoral stroma.
Homing: MSCs exhibit tropism for tumors
Tumors behave like “wounds that do not heal”, and recapitulate most of the characteristic events of damaged tissue (wounding) microenvironment, such as hypoxia, mechanical stress, sustained inflammation, and increased oxidative/nitrosative stress50,111. Numerous studies reported tumor microenvironment tropisms of both endogenous and exogenous MSCs. For instance, in a study where MSCs labeled with firefly luciferase-enhanced GFP (fLuc-eGFP) reporter gene were intravenously injected to subcutaneous and lung metastasis mouse models, the injected MSCs survived, proliferated, and differentiated in tumor sites but not anywhere else50, thereby suggesting that exogenous MSCs are disease responsive.
Various soluble molecules have been reported to play a role in the mobilization or recruitment of MSCs to tumor sites; however, the major players are the immunoregulatory cytokine TGF-β40,56,88–90, stromal cell-derived factor 1, also known as C-X-C motif chemokine 12 (CXCL12), and CXCR4, its receptor that is abundantly secreted by tumor cells118,121–124,128. Specifically, experimental evidence has established the CXCL12/CXCR4 pathway as a pivotal pathway for MSC and malignant cell migration and homing. Examples include reports suggesting the following: (i) MSC tumor tropism is mediated by matrix metalloproteinase-1 via a mechanism dependent on cross-talk with CXCL12/CXCR4 axis129 (129); (ii) CXCL12 is abundantly released by BM-MSCs and drives the homing of leukemic cells in the bone marrow stroma in pediatric precursor B-cell acute lymphoblastic leukemia130; and (iii) CXCL12/CXCR4 signals the silencing results in the inhibition of MSC migration to the primary tumor and metastasis sites in solid cancers, such as breast carcinoma119,120.
MSC paracrine activity controls stromal component production and immune response
MSCs and derived tumorigenesis-favoring cells, such as CAFs, control the production of stromal components and may sustain the maintenance of cancer cells10,11,99. For instance, MSCs regulate chemotaxis, activation, function, and survival of neutrophils via an IL-6-STAT3-ERK1/2 signaling cascade in gastric cancer and related solid cancers99,131. These MSC-primed neutrophils promote the differentiation of normal MSCs into CAFs131. Furthermore, MSCs promote angiogenic processes that result in blood vessels sustaining neoplastic tissue through its paracrine activity. The proangiogenic molecules released include IL-6, endotheline-1, VEGF, and FGF4132,133.
Unlike injured sites where tissue repair is promoted84–87,133 and in organ transplant settings where the probability of rejection of transplant tissues is decreased94,95,103, the immunosuppressive action of MSCs may result in the suppression of cancer immunity in tumors, enabling cancer cells to escape immune surveillance. As observed in tissue repair processes, MSCs can influence almost all the components of the immune system to attenuate inflammation and control immune response by interfering with various immune phenomena, such as cytokine secretion and the cytotoxicity of T- and NK cells, B-cell maturation and antibody secretion, and APC maturation, activation, and function102,134,135. Moreover, the MSC-mediated immunosuppression in the tumor stroma is partly triggered via paracrine activity. Immunosuppressive properties appear when MSCs and CAFS are involved in cell-cell interaction with immune cells, released by the anti-inflammatory cytokine TGF-β, or are stimulated by proinflammatory cytokines, such as TNF-α and IFN-γ40,56,88–90. For example, the in vitro immunosuppressive properties of MSCs towards NK, T, and B cells are triggered by the stimulation of MSCs in TNF-α and IFN-γ treatments. Such stimulation enables MSCs to produce molecules, such as PGE2, iNOS (mouse), or IDO (human)96–98. Notably, some of these products (that is, chemokines and PGE2) can attract immune cells136–138, whereas others (that is, iNOS and IDO) induce immunosuppression40,56,88–90.
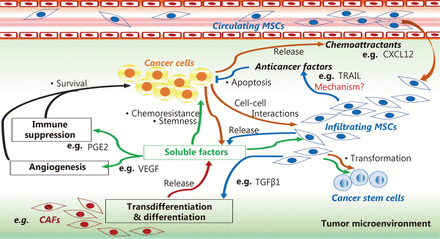
Moreover, damaged tissues and tumor microenvironments are rich in soluble factors belonging to the secretome of MSCs that can favor tumorigenic processes, such as IL-1, IL-17, IL6, IFN-γ, TNF-α, Wnt, and Jagged135,36,39–41,139. These factors can induce profound changes in the capacity for MSC, drive its differentiation into CAFs111,114,115, and produce growth factors29,35,39 and angiogenic112,115 and metastatic cytokines118–121. These findings suggest that MSCs may participate in the pathogenic vicious cycle wherein tumor cells modify stromal cells, and in turn, MSCs promote malignant cell maintenance and tumor growth via plastic and biochemical changes in the tumor microenvironment (Figure 1).
MSC role in tumor microenvironment. MSCs can induce cancer cell survival, stemness, and chemoresistance by differentiating into cancer-associated fibroblasts (CAFs) using a tumor growth factor β type 1 (TGFβ1)-dependent mechanism, and by releasing soluble factors that favor angiogenesis and immunosuppression in the tumor microenvironment, such as prostaglandin E2 (PGE2) and vascular endothelial growth factor (VEGF). MSCs can mediate anti-cancer effects by releasing anti-cancer factors, such as tumor necrosis factor-related apoptosis inducing ligand (TRAIL), via mechanisms that are not well understood.
MSC homing to tumors: pro- or antitumor action?
Discrepancies in antitumor and tumor-promoting roles of homing MSCs
Conflicting data and concepts about antitumor and tumor-promoting roles of MSCs have been reported. Most reports suggest the tumor-promoting roles of MSCs. Gastric cancer-derived MSCs can prompt gastric cancer progression through secretion of CXCL8140 and PDGF141. In another recent report, BM-MSCs protected primary B cell precursor acute lymphoblastic leukemia cells from p53 accumulation and subsequent apoptotic cell death via a PGE2-dependent mechanism142, suggesting that MSCs protect cancer cells from external aggression and confer chemoresistance10,83,102. Cross-talk between MSCs and tumor cells allows the latter to escape from apoptosis induced by chemotherapy drugs, suggesting that an enhanced understanding of such cross-talk could reveal improved targets for progressing classical therapies. Evidence-based reported mechanisms accounting for the protective interaction between MSCs and tumor cells include the activation of developmental pathways, such as Wnt, notch, sonic hedgehog, TGF-β, and MAPK83,102–105, as well as cell adhesion and growth factors10,107–109,143. Inhibiting these factors improves treatments using classical chemotherapy agents. When MSCs were cultured with B-ALL and CLL cells in presence of notch-blocking antibodies or pan notch inhibitors, like gamma-secretase inhibitors, the resistant leukemic cells were sensitized to drug-induced apoptosis, even in the presence of MSCs83,102. Similarly, the inhibition of hedgehog144 or Wnt103 signaling on MSCs enhanced the sensibility of tumors to classical chemotherapies.
The anticancer properties of MSCs have been reported, particularly the attractive MSC potential for gene or drug delivery in cancer therapy that has emerged from the cancer tropism of these cells145,146. For instance, targeted inhibition of osteosarcoma tumor growth by BM-MSCs expressing the suicide gene therapy system cytosine deaminase/5-fluorocytosine was reported in tumor-bearing mice145. Human AT-MSCs inhibited human melanoma cell growth in a conditioned medium, and a reduction in tumor size was observed in athymic mice when MSCs were injected in the tissues surrounding the tumor147. The antitumor role of MSCs was suggested by studies aimed at developing a traceable therapeutic strategy for treating breast cancer using MSCs148,149.
MSCs can induce cancer cell survival, stemness, and chemoresistance by differentiating into cancer-associated fibroblasts (CAFs) using a tumor growth factor β type 1 (TGFβ1)-dependent mechanism, and by releasing soluble factors that favor angiogenesis and immunosuppression in the tumor microenvironment, such as prostaglandin E2 (PGE2) and vascular endothelial growth factor (VEGF). MSCs can mediate anti-cancer effects by releasing anti-cancer factors, such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), via mechanisms that are not well understood.
Factors accounting for MSC ability to play both tumorigenic and anti-tumorigenic roles
Factors accounting for the ability of MSCs to play both pro- and anti-tumorigenic roles in tumor microenvironment are complex. These factors include MSC source, secretome, nature of interactions with cancer and host immune cells, type of cancer and cancer cell lines, and specific in vivo or in vitro condition7,146,150,151. Notably, MSC-secreted tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) was reported as a major promoter of MSC pro-apoptotic properties on tumor cells150,151, but its expression patterns in MSCs and cancer models has not been extensively investigated146. Consequently, data on the precise conditions of release of TRAIL and, thus, on the therapeutic relevance of the release induction by MSCs in tumors, are poorly understood, despite recent reports suggesting that MSC-released TRAIL promotes apoptosis even in resistant solid cancer cells150,151.
Moreover, concerning the origin of MSCs, several studies have been designed involving MSCs originating from healthy donors and are functionally different from cancer patients’ MSCs that have undergone deep cellular and molecular changes in the tumor stroma, following direct interaction with tumor cells111,114,115 or exposure to soluble molecules secreted by the microenvironment112–115,121. Consequently, MSCs from tumors could promote cancer progression mainly by secreting soluble factors and increasing the number of cancer-promoting stem cells in the tumor microenvironment152–154. Moreover, short-term memory of environmental stimuli and danger signals were recently reported in MSCs7, thereby increasing the complexity for predicting MSC responses in a specific environment.
Finally, discrepancies in available data emerged from changes in MSC properties when moving from in vitro to in vivo contexts155. Moreover, in a number of studies, cellular events that are considered as unequivocal indicators of antitumor or tumor promoting effect are insufficient for such conclusion. For instance, co-culture of leukemic cells with MSCs can induce growth arrest of leukemic cells; however, this cannot be always considered as an anticancer effect because cell quiescence is a well-known strategy of leukemic stem cells that allow them to escape from chemotherapeutic agents that target rapidly dividing cells156.
Conclusions
The multipotency and the ability of MSCs to secrete soluble factors that induce immunosuppression and favoring angiogenesis confer to these stem cells the ability to repair injured tissues. The specific tropism of these cells allows them to migrate and home into injured tissues to repair them and induce immunosuppression, resulting in the prevention of transplant-related immunity. These properties are the basis for the large use of MSCs in regenerative medicine, tissue engineering, and organ transplantation. However, clinical and experimental bodies of evidence show that MSCs are chemically attracted by tumors. In this context, the plastic properties of MSCs favor tumorigenesis; as soluble factors, they favor cancer cell maintenance, proliferation, chemoresistance, and suppress anticancer immunity. Moreover, MSC fusion with cancer cells and the tumor microenvironment drive MSC differentiation into CAFs, thereby favoring tumorigenesis and soluble factor release. Nevertheless, many studies showed that MSCs release potent anticancer molecules in the tumor microenvironment. Characterization of the mechanisms that drive the release of such molecules may give the cue for anticancer strategies to re-sensitize and induce apoptosis in previously chemoresistant cancer cells.
Footnotes
Conflict of interest statement No potential conflicts of interest are disclosed.
- Received March 22, 2016.
- Accepted April 6, 2017.
- Copyright: © 2017, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.
- 6.
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.
- 18.
- 19.
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.
- 48.↵
- 49.
- 50.↵
- 51.
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.
- 90.↵
- 91.↵
- 92.
- 93.
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.
- 101.↵
- 102.↵
- 103.↵
- 104.
- 105.↵
- 106.
- 107.↵
- 108.
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.
- 123.
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.↵
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.↵
- 136.↵
- 137.
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.↵
- 144.↵
- 145.↵
- 146.↵
- 147.↵
- 148.↵
- 149.↵
- 150.↵
- 151.↵
- 152.↵
- 153.
- 154.↵
- 155.↵
- 156.↵