Abstract
Primary peritoneal carcinoma (PPC) is a type of rare malignant epithelial tumor. Metastasis from PPC to breast has been rarely reported. PPC originates de novo from the peritoneal tissues rather than invasion or metastasis from adjacent or remote organs. PPCs have been implicated in many cases of carcinomas of unknown primary origin. It is similar to ovarian cancer (OvCa), because it shares the same common embryonic origin, the coelomic epithelium (mesodermal origin). The mechanism of oncogenesis remains elusive. In this article, we report a rare case of PPC in a patient 10 years after total abdominal hysterectomy and bilateral salpingooophorectomy for uterine leiomyoma, which was widely spread in the abdomen and metastasized to the colon, liver and distant organs including breast. The treatment is similar to that of primary ovarian cancer. We also reviewed the primary peritoneal cancer metastatic to breast and discuss the possible mechanisms and biology of primary peritoneal cancer, using experimental and animal model.
keywords
Introduction
Primary peritoneal carcinoma (PPC) is also known as primary serous papillary carcinoma, serous surface papillary carcinoma, extra-ovarian serous carcinoma, etc1. It originates de novo from the peritoneal tissues rather than invasion or metastasis from adjacent or remote organs. PPCs have been implicated in many cases of carcinomas of unknown primary origin. Histologically, it is similar to ovarian cancer (OvCa), because they share the same common embryonic origin, the coelomic epithelium (mesodermal origin)2, 3. The diagnosis of PPC is based on pathology and its treatment is also similar to OvCa4–6. PPC usually spreads superficially in the peritoneal cavity. It also metastasizes to liver, lung, and brain, but rarely to breast. The prognosis of PPC is usually poor with extensive cancer spread at the initial diagnosis. The pathogenesis of PPC remains illusive. In this article, we report a rare case of primary peritoneal cancer with breast metastasis in a patient 10 years after total abdominal hysterectomy and bilateral salpingo-oophorectomy.
Case report
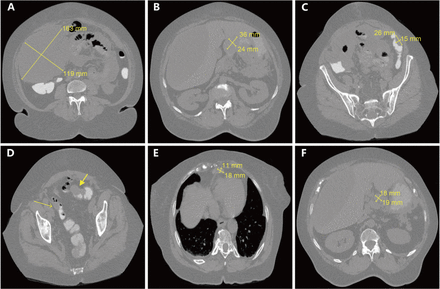
A 75-year-old African American female with PMH of uterine fibroid s/p total abdominal hysterectomy bilateral salpingo oophorectomy in 2002 presented to ER for worsening abdominal pain for 5 days and weight loss of 30 lbs in the past year. Abdominal and pelvic CT showed large volume of ascites including a large right upper quadrant (RUQ) hypodense mass around 20 cm causing compressive effects on the right lobe of the liver, a 3.6 × 2.4 cm mass-like density in anterior aspect of gastric antrum, and two 4.4 × 2.3 cm and 2.6 × 1.5 cm masses along the sigmoid colon and descending colon. Additionally, soft tissue nodularity/ infiltration along the left hemidiaphragm, spleen, pancreatic tail, small bowel loops, together with peripancreatic and mesenteric lymphadenopathy were noted, indicating neoplastic process with the peritoneal deposits (Figure 1).
CT scans of metastatic cancer with unknown primary site. (A) Large volume of intra-abdominal ascites with a large hypodense mass in the right abdomen measuring approximately 24.1 × 16.3 × 7.9 cm. (B) A 3.6 × 2.4 cm mass-like density in anterior aspect of gastric antrum. (C) A 2.6 × 1.5 cm mass on the lateral aspect of the descending colon. (D) Two masses, including a 4.4 × 2.3 cm eccentric mass along the sigmoid colon without complete obstruction. (E) A superior diaphragmatic lymphadenopathy around 1.8 × 1.1 cm. (F) Peripancreatic adenopathy measuring up to 1.9 × 1.8 cm.
The patient underwent a paracentesis. The fluid cytology analysis revealed malignant cells. As part of the workup of CT findings in the colon, a colonoscopic biopsy of the sigmoid colon was performed and showed poorly differentiated adenocarcinoma. Immunohistochemistry (IHC) staining of the colon biopsy revealed: WT1(+), CK7(+), CK20(-) and CDX2(-), phenotypically suggesting adenocarcinoma of ovary. The serum CA125 was 288 U/mL (ref. <20 U/mL). The patient subsequently underwent debulking surgery. Pathology showed positive WT1 and CK7, and negative CDX2, CEA, CK20, compatible with serous carcinoma of ovary, fallopian tube or peritoneal origin.
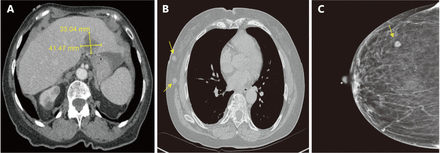
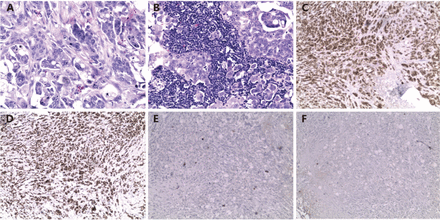
The patient completed six cycles of carboplatin/paclitaxel chemotherapy with good clinical response. A year later, abdominal CT revealed a new irregular lesion in the left lobe of the liver with thickening and nodularity of the anterior peritoneal wall, suggesting recurrent or metastatic disease. IHC stain on the tissue obtained by CT-guided biopsy was consistent with metastatic PPC. Because the patient had platinum sensitive PPC, she was then started on the second round of carboplatin/paclitaxel chemotherapy, and the left hepatic lesion remained stable. Four months later, the repeat CT revealed worsening metastatic disease demonstrated by the interval development of an additional ill-defined hepatic mass with a small new adjacent low-density lesion and two right axillary lymph nodes, measuring around 2 × 1 × 1 cm (Figure 2A and 2B). She also had mammogram at the same time and it showed a 0.8 × 0.7 × 0.8 cm hypoechoic nodule in the right breast (Figure 2C), which was biopsied under US guidance and the histology was read at an outside laboratory as an invasive ductal carcinoma, micropapillary type. Right breast lumpectomy and axillary node dissection were performed and the pathology was metastatic adenocarcinoma, primary ovarian. Both the breast mass and lymph nodes (4/10) based on morphology and IHC were consistent with PPC/serous OvCa and were positive WT1, PAX8, and CK7.
CT scans and mammogram of worsening metastatic disease since the prior study. (A) A poorly defined hypodense mass, 4.1 × 3.3 cm, in the left lobe of the liver suspicious for metastasis. (B) Two right axillary enlarged lymph nodes, measuring around 2 × 1 × 1 cm. (C) Mammogram showed a 0.8 × 0.7 × 0.8 cm nodule in the right breast on craniocaudal (CC) view.
Immunochemical and histological appearance of primary peritoneal cancer metastasized to breast. (A) Right breast lumpectomy showed metastatic breast cancer from unknown primary (H&E, 200×). (B) Metastasis was also found in right axillary node (H&E, 200×). (C) WT1 was highly expressed in metastatic breast cancer (IHC, 40×). (D) PAX8 was also highly expressed in metastatic breast cancer (IHC, 40×). (E) No expression of mammoglobin in metastatic breast cancer (IHC, 40×). (F) No expression of GATA3 in metastatic breast cancer (IHC, 40×).
Discussion
PPC is a rare malignant epithelial tumor arising from the peritoneal lining of the pelvis and abdomen. PPC is morphologically and phenotypically similar to OvCa, since they both share the same common embryonic coelomic epithelium, which is of mesonephric origin2,3. Pathogenesis of PPC remains elusive and several theories have been proposed, involving malignant transformation7, oncogenic stimulation3, unifocal origin8 and multifocal origin theories9. In some cases, PPC likely developed from embryonic germ cell exfoliated from ovary and implanted on peritoneal membrane and under the effects of a single or multiple risk factors, transformed into a malignant cell. Approximately 3.2%-21.2% of PPC patients have histories of bilateral oophorectomy for benign disease or as prophylaxis for a family history of OvCa1,4,10. This neoplasm is more common in elderly women. An initial pan-cancer marker for screening for suspected cases and biopsy is extremely important for establishing the diagnosis of PPC. Our case demonstrates the importance of routine serum test for CA125 in patients who have ascites, even in those who have prior oophorectomy for benign lesions. PPC is treated similarly as OvCa by cytoreductive surgery and chemotherapy (cisplatin/paclitaxel based). Our patient responded well to the same treatment initially. The prognosis of PPC is poor with extensive cancer spread at the initial diagnosis.
PPC usually spreads superficially in the peritoneal cavity. It also metastasizes to liver, lung, and brain, but rarely to breast. To our knowledge up to date, only two articles reported PPC with breast metastasis6,11. In one of these two reports, PPC was diagnosed 11 months after the initial diagnosis of inflammatory breast cancer11. In our case, PPC first spread to colon and liver, and clinically presented as primary colon cancer with hepatic metastasis. However, it metastasized to breast at a later stage. It still remains to be investigated how PPC metastasizes to breast. It might spread stepwisely from peritoneal adjacent organs to distal organs, or might metastasize directly through lymphatic and hematogenous pathways as per seed and soil hypothesis. Signorelli and Ghezzi12,13 have reported that BRCA1, BRCA2 or hMSH2 mutations might be related to OvCa/PPC patients with breast cancer or colon cancer. Nevertheless, it might only suggest that BRCA1, BRCA2 and hMSH2 mutations resulted in oncogeneses in different types of cells and organs. Whether they play roles in the metastases from OvCa/PPC to breast cancer remains to be investigated. Clinically, PPC is similar to ovarian cancer in its morphologic diagnosis and treatment, however, histologically it is quite different9,14. PPC is serous histology as is in our case5, while OvCa involves multiple histology types. Klein et al.15 have reported seven ovarian cancer cases metastasized to breast, among which four were serous, while the rest three were cystic, papillary, and clear cell histology. The length of survival for patients with PPC and breast metastasis is around 2 to 31 months6, with a five- year survival rate ranging from 0% to 26.5%16. Our patient lived for 36 months since first diagnosed, with good response to platinum-based chemotherapy and cytoreductive surgery.
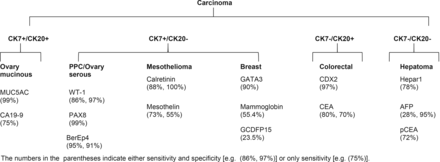
Tissue biopsy along with IHC is of paramount importance for the diagnosis of PPC since the disease has no identifiable primary lesion. Primary mesothelial carcinoma is one of the differentials that should be ruled out by negative calretinin and CK5/6. WT1 and PAX8 are valuable markers17–20 for PPC and serous OvCa. WT1 is highly sensitive and specific (86% and 97%, respectively)19,20 for PPC and serous OvCa, and PAX8 (paired box gene 8) is also highly sensitive (99%)20. However, other types of OvCa, such as mucinous OvCa, rarely express WT1 and PAX821–24. In breast cancer, GATA3 (sensibility 90%)25,26, mammaglobin (sensibility 55.4%)27 and GCDFP-15 (sensibility 74%, specificity 95%)28 are three valuable markers. GATA3 is a transcription factor that regulates the differentiation of many human tissue types, including the mammary gland. It is especially highly expressed in ER(+) and HER2(+) breast carcinomas25,26,29. Additionally, it is also expressed in mammaglobin(-)/GCDFP-15(-) TNBC29. HER2, ER and PR are also important breast cancer markers, however, they are also expressed in OvCa. While in colorectal cancer, in addition to CEA (sensibility 80%, specificity 70%), CDX2 (sensibility 97%) and CK20 are also important30,31. CK20 is often combined with CK7 to distinguish different types of cancer (Figure 4). Important liver cancer markers include HepPar1 (sensibility 78%), pCEA (sensibility 72%), AFP (sensibility 28%, specificity 95%)32. In our case, pathology from peritoneal, colon, liver and breast lesions revealed CK7(+), CK20(-), WT1(+), PAX8(+), CDX2(-), GATA3(-), mammoglobin(-), suggesting an origin from PPC or serous OvCa.
Molecular markers to distinguish undifferentiated carcinoma.
Wilms’ tumor 1 (WT1) gene, was expressed in all primary peritoneal and metastatic lesions in our case. In OvCa, it is mainly expressed in serous, endometrioid, and undifferentiated OvCa33. The characteristics of WT1 drove us to speculate its function and therapeutic value in PPC and OvCa. Cloned in 1990, WT1 is a transcription factor, serving as a tumor suppressor and oncogene34. It is widely expressed in many types of tissues and cancers35. However, its biological role in the oncogenesis of PPC and OvCa remains largely unknown. Its N-terminal proline-glutamine-rich region interacts with RNA and protein, while C-terminal zinc finger domain binds DNA. WT1 associates with numerous growth factors, receptors, transcription factors, and inhibits important tumor suppressor genes like p53, p73, thereby leading to oncogenesis34. High levels of WT1 expression are associated with higher stage of OvCa with lymph nodes and omentum metastasis by enhancing cell proliferation and invasion36, and also indicate poor survival in other cancer types19,37. Additionally, loss of heterozygosity (LOH) has also been discovered in OvCa38. Sporadic homozygous mutations are shown to lead to other cancers, such as Wilms’ tumor39. Alternative splicing, usage of alternate promoters, post-translational modification have been shown to affect WT1 expression in leukemia34. Other mechanisms such as amplification, constitutive activation, remain to be further explored. We believe that WT1 is as important as many other genes like p53, p63, KISS, and PTEN, and would play vital roles in PPC and OvCa tumorigenesis. Recently, immunotherapy targeting WT1 has been proposed as a promising therapeutics19,40. With the new development of chimeric antigen receptor T-cell therapy41, it would kindle lights on PPC and OvCa treatment.
One obstacle to study PPC oncogenesis and treatment is the lack of established animal models for PPC. However, we could use the current serous cell OvCa models to study PPC. OvCa subtype cell lines and xenografts/orthotopic implantation models are well established42. Three-dimensional culture has been developed to mimic single cell and spheroids adhering to peritoneal mesothelial cells and anchoring in the submesothelial matrix43. In addition, genetically engineered mouse models of OvCa would turn on or off specific oncogene and tumor suppressor genes in certain subtype of OvCa44. The laying hen model has also been used to mimic human spontaneous epithelial OvCa model and might also be a good model to study PPC45. The recent Cas9/gRNA and NgAgo/gDNA original genomic editing technique will potentially facilitate us to edit genes precisely in vitro and in vivo to explore the pathogenesis and treatment of PPC46.
In summary, breast metastases from primary peritoneal carcinoma have been scarcely reported. The majority of breast metastases are from the contralateral breast, and the most common extramammary sources include lung, gastrointestinal tract, lymphomas, and melanomas47. It is unknown how PPC metastasizes to breast. It may spread from both lymphatic and hematogenous pathways to seed in some organs first, such as colon and liver in our case, and finally metastasizes to breast. Distinguishing breast cancer metastasized from PPC is important because the treatment following the diagnosis would be distinct. WT1 and PAX8 are two important markers. Treatment of PPC is similar to that of OvCa. From our case, we learn an important lesson that whenever metastatic breast cancer of unknown primary origin is identified, a differential diagnosis of PPC should be considered.
Footnotes
Conflict of interest statement No potential conflicts of interest are disclosed.
- Received July 13, 2016.
- Accepted August 18, 2016.
- Copyright: © 2016, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.