Abstract
Blockade of immune checkpoints has recently emerged as a novel therapeutic strategy in various tumors. In particular, monoclonal antibodies targeting programmed cell death 1 (PD-1) or its ligand (PD-L1) have been most studied in lung cancer, and PD-1 inhibitors are now established agents in the management of non-small cell lung cancer (NSCLC). The reports on high-profile clinical trials have shown the association of PD-L1 expression by immunohistochemistry (IHC) with higher overall response rates to the PD-1/PD-L1 axis blockade suggesting that PD-L1 expression may serve as a predictive marker. Unfortunately, however, each PD-1 or PD-L1 inhibitor is coupled with a specific PD-L1 antibody, IHC protocol and scoring system for the biomarker assessment, making the head-to-head comparison of the studies difficult. Similarly, multiple clinical series that correlated PD-L1 expression with clinicopathologic and/or molecular variables and/or survival have reported conflicting results. The discrepancy could be explained by the differences in ethnicity and/or histologic types included in the studies, but it appears to be attributed in part to the differences in PD-L1 IHC methods. Thus, orchestrated efforts to standardize the PD-L1 IHC are warranted to establish the IHC as a predictive and/or prognostic biomarker in NSCLC.
keywords
Introduction
Recent advances in personalized medicine in non-small cell lung cancer (NSCLC) have dramatically shifted the paradigm of lung cancer treatment. The discovery of oncogenic driver mutations and development of the corresponding targeted agents, in particular in lung adenocarcinoma, have led to significantly improved progression free survival (PFS) for patients with advanced stage tumor harboring such a mutation1. Subsequently, patients with advanced stage lung cancer harboring an epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutation are typically treated with the corresponding tyrosin kinase inhibitor (TKI) as standard, first-line therapy2. However, most patients eventually develop resistance to genotype-specific therapies. In addition, a significant proportion of patients with NSCLC do not have genetic alterations that are currently targetable with FDA-approved therapies3.
More recently, novel therapeutic strategies, such as immunotherapy, have been investigated. Immunotherapy consists of various forms of vaccination strategies to elicit robust immune responses to tumor antigens4, and blockade of immune checkpoints to reinstitute host antitumor immunity5. Immune checkpoint molecules refer to a group of immune receptors that upon engaged with their ligands transmit an inhibitory signal to suppress effector function. While these inhibitory pathways are critical for maintaining self-tolerance and regulating the intensity and duration of immune responses in peripheral tissues to minimize tissue pathology, the same pathways can be used for cancer to evade tumor immunity5. Thus, the blockade of immune checkpoints may be effective in a variety of tumors that are refractory to other therapies. Of those, monoclonal antibodies targeting the programmed cell death-1 (PD-1, also known as CD279) receptor and its ligand, programmed cell death ligand-1 (PD-L1, also known as B7-H1) - a member of B7-family, have been most studied in the field of lung cancer. In early-phase clinical trials, PD-1 and PD-L1 inhibitors have demonstrated impressive anti-tumor activity in NSCLC6–8. In addition, randomized phase 3 trials in previously treated, advanced squamous and nonsquamous NSCLC have shown that the PD-1 inhibitor, nivolumab, leads to significant improvements in overall survival (OS) compared to single-agent docetaxel9,10. These results have led to the US Food and Drug Administration (FDA) approval of nivolumab for NSCLC patients with disease progression on or after platinum-based chemotherapy. Similarly, another anti PD-1 agent pembrolizumab has been granted accelerated US FDA approval for the treatment of patients with advanced (metastatic) NSCLC whose disease has progressed after other treatments and with tumors that express PD-L1 (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm465444.htm). The recent report on the phase 2/3 study (KEYNOTE-010) has confirmed the efficacy of pembrolizumab by demonstrating its association with significantly improved OS compared to docetaxel among patients with at least 50% of tumor cells expressing PD-L111.
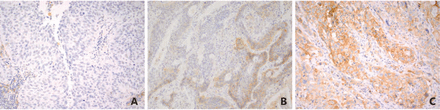
In most analyses to date, increased PD-L1 expression by immunohistochemistry (IHC) (Figure 1) has been associated with higher overall response rates to the PD-1/PD-L1 axis blockade suggesting that PD-L1 expression may serve as a predictive marker6–8,10–12. Unfortunately, such studies have invariably reported the presence of responders in patients with PD-L1 negative tumors. In addition, multiple clinical series have tired to identify clinicopathologic and/or molecular predictors of PD-L1 expression and the prognostic role of the expression, leading to the conflicting results. Thus, in this review, the clinicopathologic/molecular correlates and the predictive and prognostic value of PD-L1 expression in NSCLC will be summarized, and the issues associated with PD-L1 IHC will be discussed.
Representative images of PD-L1 immunostaining in lung squamous cell carcinoma. (A) No positive staining in the tumor cells.(B) 30% of the tumor cells with positive membranous staining. (C) The vast majority of the tumor cells with positive membranous staining.
Mechanisms of PD-L1 expression (Table 1)
To date, two different mechanisms of PD-L1 expression on tumors have been described: innate immune resistance and adaptive immune resistance5. The former represents the up-regulation of PD-L1 expression secondary to constitutive oncogenic signaling within tumor cells5. For example, Parsa et al.13 found loss of phosphatase and tensin homolog (PTEN), and the consequent activation of phosphatidylinositol-3-OH kinase (PI3K) pathway significantly increased PD-L1 expression in glioma. Similarly, Marzec et al.14 have observed that NPM-ALK rearrangements induce PD-L1 expression in anaplastic large cell lymphoma as a result of downstream activation of signal transducer and activator of transcription 3 (STAT3). Induction of PD-L1 expression has also been reported in NSCLC models harboring EGFR mutations and EML4-ALK rearrangements15,16. In particular, Chen et al.17 found that EGFR activation by EGF stimulation, exon-19 deletions, and L858R mutation could induce PD-L1 expression through p-ERK1/2/p-c-Jun but not through p-AKT/p-S6 pathway, and the induced PD-L1 expression could lead to the apoptosis of T cells through PD-1/PD-L1 axis in a co-culture system of tumor cells and peripheral blood mononuclear cells obtained from healthy volunteers. Furthermore, PD-L1 expression was reduced in these models following treatment with the corresponding TKIs. In clinical studies, several reports suggested that EGFR mutations and ALK rearrangements were associated with PD-L1 expression15,16 with up to 72% of EGFR-mutant patients18 and 78% of ALK-rearranged patients19 exhibiting positive expression. In the study with 56 EGFR-mutated advanced lung adenocarcinomas, PD-L1 expression was associated with greater disease-control rate (P=0.004) and longer PFS (P=0.001) after EGFR TKI therapy and longer OS (P=0.004)20. Other studies failed to find the association between PD-L1 expression and these oncogenic drivers, however21.
Mechanisms of PD-L1 expression on tumor cells
By contrast, in adaptive immune resistance, PD-L1 expression is induced on tumor cells secondary to local inflammatory signals. When tumor antigen-specific T cells recognize their cognate antigen expressed by cancer cells, signaling through the T-cell receptor leads to the expression of activation-induced regulatory receptors, including PD-1 as well as the production of interferons that are aimed at amplifying the immune response and attracting other immune cells such as macrophages22. However, the interferons, in particular interferon γ, leads to expression of PD-L1 on tumor cells and/or inflammatory cells including T cells, NK cells, monocytes/macrophages, dendritic cells, B cells and/or others23, likely via the canonical type 2 interferon receptor signaling24. When engaged by PD-L1 or the other ligand PD-L2, PD-1 inhibits kinases that are involved in T cell activation through the phosphatase SHP250 leading to apoptosis of T cells, although addi tional signaling pathways are likely also induced5,25,26. The observation that PD-L1 expression is often restricted in T cell-rich areas of tumors, in particular at the invasive margin, supports the presence of adaptive immune resistance in most cancer histologies27,28. In this setting, blockade of PD-1/PD-L1 interaction will reinstitute the active antitumor immune response.
It is yet determined whether the mechanism underlying tumor PD-L1 expression (i.e. innate vs. adaptive immune resistance) impacts responsiveness to PD-1/PD-L1 inhibitors in the clinic. However, clinical trial data have suggested that EGFR-mutant patients have only modest response rates to PD-1 blockade. For example, in the phase 3 randomized CheckMate 057 trial with previously-treated non-squamous NSCLC patients, the PD-1 inhibitor nivolumab led to a significant improvement in OS compared to docetaxel; however, there was no difference between the two study arms among a subset of EGFR-mutant patients10.
Other mechanisms for up-regulation of PD-L1 expression include simultaneous amplifications of PD-L1 and JAK2, both of which are located in the chromosome 9p21 region29, up- or down-regulation of micro RNAs30–32, and hypoxia (through the production of hypoxia inducible factor 1α, HIF1α)33,34. For instance, up-regulation of miR-20b, -21, and -130b have been shown to result in PD-L1 expression through down-regulation of PTEN expression in colorectal cancer30, while miR-200 suppresses the expression of PD-L1 on tumor cells that is restituted by an epithelial-mesenchymal transformation activator, ZEB132. Furthermore, miR-197 that is often down-regulated in chemoresistant NSCLC suppresses cyclin-dependent kinase CKS1B that facilitates phosphorylation of STAT3 leading to PD-L1 expression as well as transcription of Bcl-2, c-Myc and cyclin D131. Thus, down-regulation of miR-200 and miR-197 is associated with PD-L1 expression. As for hypoxia-related PD-L1 expression, HIF1α reportedly binds to a hypoxia-response element in the PD-L1 proximal promoter34.
PD-L1 expression and clinicopathologic and molecular correlation in NSCLC
The recent clinical series reported various extents of PD-L1 expression in NSCLC raging from 7.4% to 72.7%. Similarly the same series attempted to correlate PD-L1 expression with clinicopathologic parameters and/or molecular alterations leading to conflicting results (Table 2)18–21,35–47. In order to overcome potential bias due to the limited sample size of each cohort, a meta analysis including nine of the above studies with 10 cohorts consisting of 1,550 NSCLC patients was conducted and showed that, among several clinicopathologic features, only poor differentiation of tumor was a significant predictor of PD-L1 expression, and positive smoking history was marginally associated with PD-L1 expression48.
Correlation of PD-L1 expression with clinicopathologic and molecular variables and prognosis
Now, multiple studies in various malignancies have shown that PD-L1 expression is linked to significant tumor infiltrating lymphocytes (TILs) in the tumor microenvironment25,42,46. Several studies with NSCLC cohorts have included the analysis of TILs on routine stain and/or IHC in association with PD-L1 expression, again leading to conflicting results19,36,37,42,43,45,46, although more recent studies have shown positive association of PD-L1 expression on tumor cells with increased TILs (Table 3). For instance, tumors with positive PD-L1 expression by immunofluorescence (AQUA) exhibited prominent (grade 2-3) TILs on routine histology in both Greek and US cohorts46. In the study using Aperio system for counting intratumoral CD8+ or PD-1+ immune cells, PD-L1 expression was significantly associated with increased CD8+ TILs, but not with increased PD-1+ TILs in a squamous carcinoma cohort42. Interestingly, these studies also reported increased TILs as a predictor of improved patient outcomes19. Similarly, in our resent study with a cohort of 242 lung adenocarcinomas, we demonstrated the association of PD-L1 expression on tumor cells with increased CD8+ and/or T-bet+ (a Th1 pathway transcription factor) TILs as well as a predictive role of increased CD8+ TILs for both improved recurrence-free survival and OS49. Of note, we used PD-L1 IHC with clone E3L1N (Cell Signaling Technology, Danvers, MA, USA) and Leica automation (Leica Microsystems, Bannockburn, IL), and the PD-L1 expression was considered positive if membranous +/- cytoplasmic staining intensity was present in 5% or more of tumor cells49.
Tumoral immune cell infiltration in association with PD-L1 expression on tumor cells and prognosis
Given that the limited number of studies included molecularly annotated cohorts, a meta analysis to evaluate correlation of PD-L1 expression with molecular alterations has not been conducted. As mentioned above, some studies have reported the association of PD-L1 expression with EGFR mutations, or EGFR protein overexpression (in squamous cell carcinomas) but others did not find the association16,18,21,35. Interestingly, the recent report on an early-phase clinical trial of pembrolizumab for the treatment of NSCLC has shown no difference in PD-L1 expression between EGFR mutants and EGFR wild-type tumors (18/54 vs. 95/288, P=1.000) in a subset analysis, while there was significant association between PD-L1 expression and KRAS mutations. Of 52 tumors harboring a KRAS mutation 44.2% exhibited PD-L1 expression in 50% or greater of the tumor cells, while 26.8% of 157 KRAS wild-type tumors were positive for PD-L1 overexpression (P=0.0003)6. Similarly, in the study evaluating PD-L1 expression in 60 NSCLC tumor samples using IHC with clone 28-8 and a cut-off of 5% (>5%) for positivity, Harbison et al.50 found that 42% of the tumor samples were positive for PD-L1 expression. Of those 53 tumors were tested for KRAS mutations, and PD-L1 expression was associated with the presence of KRAS mutations (8/10 vs. 15/43 with negative KRAS mutations, P=0.014). Furthermore, by a gene expression analysis using an Affymetrix platform they revealed sharp demarcation of PD-L1 gene expression between PD-L1 IHC positive and negative tumors, and overexpression of several immune-related genes (e.g. interferon γ) and other genes involved in immune-cell regulation, tumor progression or signaling pathways (e.g. MET) in PD-L1 IHC positive tumors.50 In the aforementioned study of ours, a subset analysis of a molecularly annotated cohort (n=128) revealed association of PD-L1 expression and KRAS mutations and their associated features, including smoking history and solid predominant pattern of histology. Notably, 38% of KRAS mutants demonstrated both PD-L1 expression and increased CD8+ TILs, while only 5.1% of non-KRAS mutants exhibited concurrent PD-L1 and increased CD8+ TILs, and none of those had driver alterations identified by clinical molecular testing49.
These results suggest the presence of acquired immune resistance in at least a subset of KRAS-mutated NSCLC, and blockade of the PD-1/PD-L1 axis may be a promising treatment strategy for those tumors. In fact, patients with a KRAS-mutated tumor more likely experienced benefits from treatment with nivolmab as shown in the phase 3 clinical trial on nivolumab vs. docetaxel in advanced nonsquamous NSCLC10.
Prognostic role of PD-L1 expression in NSCLC
The previously mentioned meta analysis has shown the association of PD-L1 expression with reduced OS (HR=1.47; 95% CI: 1.19-1.83; P=0.0004)48, while a more recently conducted meta analysis including 11 of the studies with 12 cohorts consisting of 1, 653 NSCLC patients failed to show a role of PD-L1 expression in predicting OS (HR=1.21, 95% CI: 0.85-1.71, P=0.29)51. Of note, the majority of cohorts included in the two studies consisted of Chinese patients, and a subgroup analysis showed a significant association between PD-L1 expression and reduced OS in Chinese patients (HR=1.55; 95% CI: 1.04-2.29, P=0.03) in the latter study. Thus, the conflicting results may be due in part to difference in ethnicity. In addition, the differences in PD-L1 antibody clones, IHC protocols and scoring systems used in the various studies (Table 4) could contribute to the conflicting results. In fact, positive PD-L1 expression determined by IHC using rabbit and/or polyclonal antibodies were associated with reduced OS in the aforementioned meta analysis51.
Comparison of PD-L1 IHC evaluations
PD-L1 expression as a predictive marker
As mentioned earlier, a series of high profile clinical trials demonstrated the benefit of PD-1 inhibitors pembrolizumab in advanced NSCLC and nivolumab in advanced squamous and nonsquamous NSCLC, and subsequently both agents have been approved as second line therapies by FDA6,9,10. PD-L1 inhibitors atezolizumab and durvalumab have also demonstrated efficacy in various tumors including NSCLC12,52. Although only preliminary clinical data is available on these PD-L1 inhibitors to date, it is possible that those agents will be granted FDA approval in 2016.Notably, membranous +/- cytoplasmic expression of PD-L1 in targeted cells demonstrated by IHC appears associated with response to PD-1/PD-L1 inhibitors (Figure 2)6,10,12,52. Garon et al.6showed that patients whose tumors had PD-L1 expression in >50% tumor cells were significantly more likely to respond to pembrolizumab than those with <50% tumor cell expression. Among all the patients, the objective response rate was 19.4%, and the median PFS and OS were 3.7 months and 12.0 months, respectively, while among the patients with PD-L1 expression in >50% tumor cells, the overall response rate was up to 45.2%, and the median PFS and OS were 6.3 months and not reached, respectively. Their study applied the Dako PD-L1 IHC 22C3 pharmDx test on the Autostainer Link 48 (Dako, Carpinteria, CA), and this combination of antibody clone and detection system was approved by FDA as a companion diagnostic to select NSCLC patients for treatment with pembrolizumab ( Table 5)6. Similarly, in the phase 3 study comparing efficacy of nivolumab and docetaxel in previously treated, advanced nonsquamous NSCLC, nivolumab was associated with longer OS and PFS and higher objective response rates than docetaxel in the groups of patients whose tumors exhibited PD-L1 expression levels of >1%, >5%, and >10%, but not in patents with PD-L1 expression in <1% of tumor cells (31% vs. 9% for the >1% group and 12% vs. 15% for the <1% group), indicating that PD-L1 expression enriches for responders 10. Of note, PD-L1 expression did not predict differential response to nivolumab in lung squamous cell carcinoma as compared to docetaxol9. In the nivolumab trials, PD-L1 IHC was performed with the same Dako detection system but a different antibody clone (28-8, Abcam, Cambridge, MA) (Table 5)10. As for the response to PD-L1 inhibitors, in the recent report on the phase 2 clinical trial (POPLAR) comparing atezolizumab (MPDL3280A) and docetaxel in previously treated NSCLC patients, atezolizumab treatment led to improved OS (HR 0.63, 95% CI 0.42-0.94, P=0.024) in the group with positive PD-L1 expression, but not in the PD-L1 negative group (HR 0.70, 95%IC 0.64-1.93, P=0.70)12. Of note, the evaluation of PD-L1 expression appears more complex in atezolizumab trials since the expression in both tumor cells and intratumoral immune cells are taken into account (Table 5). There is no mature information available for durvalumab yet, but the preliminary results of the phase 1/2 clinical trial indicate the association of PD-L1 expression with likelihood of response to the agent52. The absence of expression, however, is not an absolute indicator of the lack of response, since a small fraction of patients with PD-L1 negative tumors also responded to the PD-1/PD-L1 agent in all the above trials. Thus, the predictive value of PD-L1 IHC is not at the same level as that of molecular testing for EGFR mutations or ALK rearrangements.
PD-L1 expression and response to PD-1/PD-L1 inhibitors in NSCLC.
PD-L1 IHC assays applied in clinical trials
Now, accumulating evidence suggests immunologic effects of platinum chemotherapeutics on the tumor microenvironment that enhance anti-tumor T cell immunity due in part to down-regulation of PD-1 pathway. Thus positive PD-L1 expression may serve as a predictor of response to platinum-based chemotherapy not only in advanced NSCLC but also in early stage tumors. The possible immunologic effects by platinum agents include: 1) attraction of dendritic cells through ATP released from tumor cells dying from platinum exposure and phagocytosis of dying cells with expression of calreticulin on their surface by the dendritic cells; 2) the extracellular ATP, together with high mobility group box-1 (HMGB-1), leading to dendritic cell maturation and upregulation of costimulatory molecules and presentation of tumor-specific peptides on MHC class I; 3) the maturation of dendritic cells in the presence of platinum drugs resulting in downregulation of PD-L1 and PD-L2 on the dendritic cells, increasing their T-cell activation potential; 4) inactivation of STAT6 in the tumor cells, leading to decreased PD-L2 expression, resulting in enhanced recognition and killing by the tumor-specific T cells; 5) upregulation of M6P receptor on tumor cells leading to enhanced tumor cell lysis by granzyme-B secreted by the activated T cells53. Tumor cells with PD-L1 expression are often present in association with cytotoxic T cell/Th1 microenvironment, thus they may be more sensitive to platinum-based chemotherapies since the platinum chemotherapeutics could restitute the immune microenvironment. In a study with resected stage 2-3 lung adenocarcinomas, we have shown improved recurrence-free survival in patients with PD-L1 positive tumor compared to those with PD-L1 negative tumor after treatment with platinum-based adjuvant therapy, supporting the hypothesis54. The recent study with stage 3 NSCLC patients who underwent concurrent chemoradiation therapy found no correlation between PD-L1 expression and OS or PFS, however55. Although the discrepant results could be explained by the differences in treatment modalities (i.e. chemotherapy only in the adjuvant setting vs. concurrent chemoradiation therapy), ethnicity and PD-L1 IHC methods, larger-scale, prospective studies are warranted to determine the predictive role of PD-L1 expression in chemotherapy/chemoradiation therapy settings.
Issues associated with PD-L1 IHC
As discussed earlier, the clinical series reported conflicting results on clinicopathologic and/or molecular characteristics as well as survival of NSCLC with PD-L1 expression. It is attributed in part to the difference in ethnicity of the cohorts, but it could also be explained by the diversity of PD-L1 antibody clones and plethora of detection systems (Table 4). Several companies have developed different primary antibodies against PD-L1 protein consisting of monoclonal or polyclonal antibodies and those targeting the intracellular or extracellular domain56. Of those, four monoclonal antibodies, 22C3, 22-8, SP142 and SP263, that are used in a biomarker assay in the main clinical trials for PD-1/PD-L1 inhibitors have been most vigorously validated (Table 5). To date, however, there has been no head-to-head comparison of sensitivity for PD-L1 expression between the four clones. In order to elucidate the issue of interassay concordance, McLaughlin, et al.57 recently compared the extent of PD-L1 expression between SP142 and one of the most carefully validated non-trial monoclonal antibodies, E1L3N, by quantitative immunofluorescence, and showed significant discordance in the expression between the two clones (25% in 588 serial section fields). Of note, both SP142 and E1L3N are against the intracellular domain of PD-L1. Furthermore, clinical trials for each PD-1 or PD-L1 inhibitor apply not only a specific antibody coupled with the specific detection system but also a specific scoring method/cut-offs (Table 5). The absence of universally accepted reference standard for PD-L1 IHC and its interpretation makes comparison of the results of clinical studies and trials extremely difficult. Thus, efforts to standardize the IHC protocol or at least to compare performance of the assays with respect to targets, intensities, frequency of staining, etc. are warranted (http://www.aacr. org/AdvocacyPolicy/GovernmentAffairs/Pages/industry-working-group-blueprint-proposal.aspx#.VpxhtLTWuCs).
Another important issue is intratumoral heterogeneity in PD-L1 expression. In the McLaughlin study, the concordance of PD-L1 expression between the tissue microarray core and the corresponding whole tumor section in 49 NSCLC cases was not significant57. Ilie et al.58 recently compared PD-L1 expression using clone SP149 between the preoperative biopsy and the corresponding resections in 160 NSCLC patients and found significant discordance between the two (the overall discordance rate=48% and κ value=0.218). Interestingly, the discordance was mainly attributed to negative or reduced immune cell scores in the biopsies compared to those in the resection specimens. Given that immune cell infiltration is often focal in the tumor area, PD-L1 IHC interpretation that includes the evaluation of immune cells appears to be more sensitive to the heterogeneity of PD-L1 expression. As mentioned earlier, however, all the biomarker assessments of the four clinical trial antibody clones have reported a small fraction of patients with PD-L1 negative tumors responding to anti PD-1/PD-L1 agents, and it could be explained by the underdetection of PD-L1 expression in the biopsy sample from advanced NSCLC due to intratumoral heterogeneity.
Similarly, intertumoral heterogeneity in PD-L1 expression is not insignificant. Kim and colleagues analyzed 331 resected pulmonary squamous cell carcinomas and showed that PD-L1 expression status maintained in 70.3% of metastatic regional lymph nodes, while PD-L1 expression was present in the primary tumor and absent in the metastatic lymph node in 18.9%, and the reverse was true in 10.8%42. We also observed the similar discrepant rate (25%) between the primary tumor and nodal metastasis as well as between nodal metastases in stage 2 and 3 lung adenocarcinomas54. These results raise a concern about selecting the most appropriate tissue sample for assessment of PD-L1 expression that will determine the eligibility for treatment with anti-PD-1/PD-L1 agents.
Finally, Kim et al.59 have reported that significant paired samples obtained from the patient at different time points (a mean interval of 20.9 months) showed discrepant PD-L1 expression by IHC using the 22C3 clone suggesting dynamic changes in PD-L1 expression in the given tumor. Thus, it may be important to assess PD-L1 expression in a newly procured tissue sample before treatment with PD-1/PD-L1 inhibitors.
Other possible markers to predict response to anti-PD-1/PD-L1 agents
Given the not perfect negative predictive value of PD-L1 IHC, additional biomarkers in selecting patients for treatment with anti-PD-1/PD-L1 agents are warranted. The study by Rivzi et al.60 has shown the association of improved objective response, durable clinical benefits and PFS with higher nonsynonymous mutation burdens in tumors depicted by whole-exome sequencing in NSCLC treated with pembolizumab. The efficacy also correlated with the molecular smoking signature, higher neoantigen burden and DNA repair pathway mutations. Similarly, Ribas et al.61 has reported the utility of nanostring profiling of INFγ signaling markers, antigen presenting machinery and T-cell-specific makers in predicting response to PD-1 blockade with pembrolizumab in melanoma patients. These genes/markers will likely be rigorously validated using clinical trial samples and/or those from clinically treated patients since the advance in technology has made these rather sophisticated methods feasible for formalin-fixed paraffin-embedded tissue samples. Given the hypothesis that the response to PD-1/PD-L1 axis blockade occurs in patients with a pre-existing INF-mediated adaptive immune response, the demonstration of cytotoxic T cell/Th1 immune environment (CD8+ and/or a Th1 transcription factor, T-bet) by IHC and/or the detection of increased INFγ in the tumor tissue by in situ hybridization (ISH) may be proven useful.
Conclusions
Both clinicopathologic studies and clinical trials evaluating PD-L1 expression in NSCLC have used various PD-L1 IHC methods including antibody clones, IHC protocols, target cell types and cut-offs for positivity, and have led to conflicting results and difficulty in the head-to-head comparison of efficacy between various anti-PD-1/PD-L1 agents, respectively. Thus, orchestrated efforts to standardize the IHC protocol or at least to compare performance of the assays with respect to targets, intensities, frequency of staining, etc. are warranted to establish PD-L1 expression by IHC as a predictive and prognostic biomarker in NSCLC. In addition, the issues of intratumoral, intertumoral and temporal heterogeneity of PD-L1 expression should be addressed to identify the best sample to conduct PD-L1 IHC. Finally, given the not perfect negative predictive value of PD-L1 expression, additional biomarkers in selecting patients for treatment with anti-PD-1/PD-L1 agents need to be explored.
Acknowledgements
This work was supported by a Stand Up To Cancer-American Cancer Society Dream Team Translation Research Grant. The author thanks Ms. Tiffany Huynh and Ms. Cris Kenudson for editorial supports.
Footnotes
Conflict of interest statement Mari Mino-Kenudson has served as a consultant for Merrimack Pharmaceuticals.
- Received January 19, 2016.
- Accepted February 22, 2016.
- Copyright: © 2016, Cancer Biology & Medicine
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY) 4.0, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.