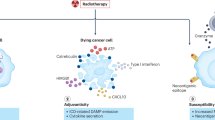
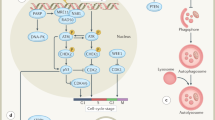
Key Points
-
Radiotherapy needs a paradigm shift to include biological interventions that are tailored to radiation-related phenomena
-
DNA-repair mechanisms are obvious targets for interventions aimed at improving the radiotherapeutic benefit
-
Chromatin structure and nuclear architecture critically influence the dynamics and extent of DNA damage and repair, and thus the response to radiation
-
Cells exist along a wide spectrum of radiation responsiveness, with cancer stem cells generally being radioresistant
-
Radiation therapy can be an antitumour immune adjuvant and new approaches to immunotherapy will offer the opportunity to exploit this interaction
-
Biomarkers that are redox-related or immune-related might help evaluate the status of patients with cancer and provide insight into how best to combine radiotherapy with biological treatments in each individual
Abstract
The past 20 years have seen dramatic changes in the delivery of radiation therapy, but the impact of radiobiology on the clinic has been far less substantial. A major consideration in the use of radiotherapy has been on how best to exploit differences between the tumour and host tissue characteristics, which in the past has been achieved empirically by radiation-dose fractionation. New advances are uncovering some of the mechanistic processes that underlie this success story. In this Review, we focus on how these processes might be targeted to improve the outcome of radiotherapy at the individual patient level. This approach would seem a more productive avenue of treatment than simply trying to increase the radiation dose delivered to the tumour.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Thariat, J., Hannoun-Levi, J. M., Sun Myint, A., Vuong, T. & Gerard, J. P. Past, present, and future of radiotherapy for the benefit of patients. Nat. Rev. Clin. Oncol. 10, 52–60 (2013).
Loeffler, J. S. & Durante, M. Charged particle therapy–optimization, challenges and future directions. Nat. Rev. Clin. Oncol. 10, 411–424 (2013).
Lo, S. S. et al. Stereotactic body radiation therapy: a novel treatment modality. Nat. Rev. Clin. Oncol. 7, 44–54 (2010).
Withers, H. R. The 4Rs of radiotherapy in Advances in Radiation Biology Vol. 5 (eds Lett, J. T. & Alder, H.) 241–249 (New York: Academic Press, 1975).
Steel, G. G., McMillan, T. J. & Peacock, J. H. The 5Rs of radiobiology. Int. J. Radiat. Biol. 56, 1045–1048 (1989).
Good, J. S. & Harrington, K. J. The hallmarks of cancer and the radiation oncologist: updating the 5Rs of radiobiology. Clin. Oncol. (R. Coll. Radiol.) 25, 569–577 (2013).
Helleday, T., Petermann, E., Lundin, C., Hodgson, B. & Sharma, R. A. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 8, 193–204 (2008).
Chinnaiyan, P., Allen, G. W. & Harari, P. M. Radiation and new molecular agents, part II: targeting HDAC, HSP90, IGF-1R, PI3K, and Ras. Semin. Radiat. Oncol. 16, 59–64 (2006).
Rizvi, N. A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Fuks, Z. & Kolesnick, R. Engaging the vascular component of the tumor response. Cancer Cell 8, 89–91 (2005).
Masterson, L. et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cochrane Database of Systematic Reviews, Issue 2. Art. No.: CD010271 http://dx.doi.org/10.1002/14651858.CD010271.pub2 (2014).
Lomax, M. E., Folkes, L. K. & O'Neill, P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 25, 578–585 (2013).
Kastan, M. B., Onyekwere, O., Sidransky, D., Vogelstein, B. & Craig, R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51, 6304–6311 (1991).
Rogakou, E. P., Pilch, D. R., Orr, A. H., Ivanova, V. S. & Bonner, W. M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 (1998).
Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009).
Wang, X. et al. Chk1-mediated phosphorylation of FANCE is required for the Fanconi anemia/BRCA pathway. Mol. Cell Biol. 27, 3098–3108 (2007).
Bekker-Jensen, S. & Mailand, N. Assembly and function of DNA double-strand break repair foci in mammalian cells. DNA Repair (Amst.) 9, 1219–1228 (2010).
Lieber, M. R. NHEJ and its backup pathways in chromosomal translocations. Nat. Struct. Mol. Biol. 17, 393–395 (2010).
Lieber, M. R. & Wilson, T. E. SnapShot: nonhomologous DNA end joining (NHEJ). Cell 142, 496–496.e1 (2010).
San Filippo, J., Sung, P. & Klein, H. Mechanism of eukaryotic homologous recombination. Annu. Rev. Biochem. 77, 229–257 (2008).
Onn, I., Heidinger-Pauli, J. M., Guacci, V., Unal, E. & Koshland, D. E. Sister chromatid cohesion: a simple concept with a complex reality. Annu. Rev. Cell Dev. Biol. 24, 105–129 (2008).
Maréchal, A. & Zou, L. RPA-coated single-stranded DNA as a platform for post-translational modifications in the DNA damage response. Cell Res. 25, 9–23 (2014).
Moore, S., Stanley, F. K. & Goodarzi, A. A. The repair of environmentally relevant DNA double strand breaks caused by high linear energy transfer irradiation--no simple task. DNA Repair (Amst.) 17, 64–73 (2014).
Takahashi, A. et al. Nonhomologous end-joining repair plays a more important role than homologous recombination repair in defining radiosensitivity after exposure to high-LET radiation. Radiat. Res. 182, 338–344 (2014).
Averbeck, N. B. et al. DNA end resection is needed for the repair of complex lesions in G1-phase human cells. Cell Cycle 13, 2509–2516 (2014).
Durante, M. New challenges in high-energy particle radiobiology. Br. J. Radiol. 87, 20130626 (2014).
Nakajima, N. I. et al. Pre-exposure to ionizing radiation stimulates DNA double strand break end resection, promoting the use of homologous recombination repair. PLoS ONE 10, e0122582 (2015).
Shrivastav, M., De Haro, L. P. & Nickoloff, J. A. Regulation of DNA double-strand break repair pathway choice. Cell Res. 18, 134–147 (2008).
Hufnagl, A. et al. The link between cell-cycle dependent radiosensitivity and repair pathways: A model based on the local, sister-chromatid conformation dependent switch between NHEJ and HR. DNA Repair (Amst.) 27, 28–39 (2015).
Aylon, Y., Liefshitz, B. & Kupiec, M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 23, 4868–4875 (2004).
Hentges, P., Waller, H., Reis, C. C., Ferreira, M. G. & Doherty, A. J. Cdk1 restrains NHEJ through phosphorylation of XRCC4-like factor Xlf1. Cell Rep. 9, 2011–2017 (2014).
Sorensen, C. S. et al. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat. Cell Biol. 7, 195–201 (2005).
Bryant, C., Scriven, K. & Massey, A. J. Inhibition of the checkpoint kinase Chk1 induces DNA damage and cell death in human leukemia and lymphoma cells. Mol. Cancer 13, 147 (2014).
Sears, C. R. & Turchi, J. J. Complex cisplatin-double strand break (DSB) lesions directly impair cellular non-homologous end-joining (NHEJ) independent of downstream damage response (DDR) pathways. J. Biol. Chem. 287, 24263–24272 (2012).
Gibson, B. A. & Kraus, W. L. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat. Rev. Mol. Cell Biol. 13, 411–424 (2012).
Noel, G. et al. Radiosensitization by the poly(ADP-ribose) polymerase inhibitor 4-amino-1,8-naphthalimide is specific of the S phase of the cell cycle and involves arrest of DNA synthesis. Mol. Cancer Ther. 5, 564–574 (2006).
Audeh, M. W. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376, 245–251 (2010).
Fong, P. C. et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009).
Koppensteiner, R. et al. Effect of MRE11 loss on PARP-inhibitor sensitivity in endometrial cancer in vitro. PLoS ONE 9, e100041 (2014).
Wang, L. et al. MK-4827, a PARP-1/-2 inhibitor, strongly enhances response of human lung and breast cancer xenografts to radiation. Invest. New Drugs 30, 2113–2120 (2012).
Reiss, K. A. et al. A phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy in patients with advanced solid malignancies and peritoneal carcinomatosis. Clin. Cancer Res. (2014).
Chow, J. P. et al. PARP1 is overexpressed in nasopharyngeal carcinoma and its inhibition enhances radiotherapy. Mol. Cancer Ther. 12, 2517–2528 (2013).
Nowsheen, S., Bonner, J. A. & Yang, E. S. The poly(ADP-Ribose) polymerase inhibitor ABT-888 reduces radiation-induced nuclear EGFR and augments head and neck tumor response to radiotherapy. Radiother. Oncol. 99, 331–338 (2011).
Chatterjee, P. et al. PARP inhibition sensitizes to low dose-rate radiation TMPRSS2–ERG fusion gene-expressing and PTEN-deficient prostate cancer cells. PLoS ONE 8, e60408 (2013).
Castri, P. et al. Poly(ADP-ribose) polymerase-1 and its cleavage products differentially modulate cellular protection through NF-κB-dependent signaling. Biochim. Biophys. Acta 1843, 640–651 (2014).
Hunter, J. E. et al. NF-κB mediates radio-sensitization by the PARP-1 inhibitor, AG-014699. Oncogene 31, 251–264 (2012).
Feng, F. Y. et al. Targeted radiosensitization with PARP1 inhibition: optimization of therapy and identification of biomarkers of response in breast cancer. Breast Cancer Res. Treat. 147, 81–94 (2014).
O'Shaughnessy, J. et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N. Engl. J. Med. 364, 205–214 (2011).
Patel, A. G., De Lorenzo, S. B., Flatten, K. S., Poirier, G. G. & Kaufmann, S. H. Failure of iniparib to inhibit poly(ADP-ribose) polymerase in vitro. Clin. Cancer Res. 18, 1655–1662 (2012).
Morgan, M. A., Parsels, L. A., Maybaum, J. & Lawrence, T. S. Improving the efficacy of chemoradiation with targeted agents. Cancer Discov. 4, 280–291 (2014).
Takata, H. et al. Chromatin compaction protects genomic DNA from radiation damage. PLoS ONE 8, e75622 (2013).
Chapman, J. D. et al. Condensed chromatin and cell inactivation by single-hit kinetics. Radiat. Res. 151, 433–441 (1999).
Chiolo, I. et al. Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell 144, 732–744 (2011).
Storch, K. et al. Three-dimensional cell growth confers radioresistance by chromatin density modification. Cancer Res. 70, 3925–3934 (2010).
Jakob, B. et al. DNA double-strand breaks in heterochromatin elicit fast repair protein recruitment, histone H2AX phosphorylation and relocation to euchromatin. Nucleic Acids Res. 39, 6489–6499 (2011).
Chiolo, I., Tang, J., Georgescu, W. & Costes, S. V. Nuclear dynamics of radiation-induced foci in euchromatin and heterochromatin. Mutat. Res. 750, 56–66 (2013).
Jezkova, L. et al. Function of chromatin structure and dynamics in DNA damage, repair and misrepair: gamma-rays and protons in action. Appl. Radiat. Isot. 83 (Pt B), 128–136 (2014).
Falk, M., Lukasova, E. & Kozubek, S. Higher-order chromatin structure in DSB induction, repair and misrepair. Mutat. Res. 704, 88–100 (2010).
Frame, F. M. et al. HDAC inhibitor confers radiosensitivity to prostate stem-like cells. Br. J. Cancer 109, 3023–3033 (2013).
Pugh, J. L. et al. Histone deacetylation critically determines T cell subset radiosensitivity. J. Immunol. 193, 1451–1458 (2014).
Kruhlak, M. J., Celeste, A. & Nussenzweig, A. Monitoring DNA breaks in optically highlighted chromatin in living cells by laser scanning confocal microscopy. Methods Mol. Biol. 523, 125–140 (2009).
Becker, A., Durante, M., Taucher-Scholz, G. & Jakob, B. ATM alters the otherwise robust chromatin mobility at sites of DNA double-strand breaks (DSBs) in human cells. PLoS ONE 9, e92640 (2014).
Goodarzi, A. A., Jeggo, P. & Lobrich, M. The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax. DNA Repair (Amst.) 9, 1273–1282 (2010).
Goodarzi, A. A. & Jeggo, P. A. The heterochromatic barrier to DNA double strand break repair: how to get the entry visa. Int. J. Mol. Sci. 13, 11844–11860 (2012).
Iyengar, S. & Farnham, P. J. KAP1 protein: an enigmatic master regulator of the genome. J. Biol. Chem. 286, 26267–26276 (2011).
Lee, D. H. et al. Phosphoproteomic analysis reveals that PP4 dephosphorylates KAP-1 impacting the DNA damage response. EMBO J. 31, 2403–2415 (2012).
Dimitrova, N., Chen, Y. C., Spector, D. L. & de Lange, T. 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature 456, 524–528 (2008).
Averbeck, N. B. & Durante, M. Protein acetylation within the cellular response to radiation. J. Cell Physiol. 226, 962–967 (2011).
Rosato, R. R. & Grant, S. Histone deacetylase inhibitors in cancer therapy. Cancer Biol. Ther. 2, 30–37 (2003).
Zhang, L. et al. Recent progress in the development of histone deacetylase inhibitors as anti-cancer agents. Mini Rev. Med. Chem. 13, 1999–2013 (2013).
Camphausen, K. et al. Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int. J. Cancer 114, 380–386 (2005).
Cerna, D., Camphausen, K. & Tofilon, P. J. Histone deacetylation as a target for radiosensitization. Curr. Top. Dev. Biol. 73, 173–204 (2006).
Shabason, J. E., Tofilon, P. J. & Camphausen, K. HDAC inhibitors in cancer care. Oncology (Williston Park) 24, 180–185 (2010).
Jung, M. et al. Novel HDAC inhibitors with radiosensitizing properties. Radiat. Res. 163, 488–493 (2005).
Konsoula, Z., Velena, A., Lee, R., Dritschilo, A. & Jung, M. Histone deacetylase inhibitor: antineoplastic agent and radiation modulator. Adv. Exp. Med. Biol. 720, 171–179 (2011).
Chinnaiyan, P. et al. Postradiation sensitization of the histone deacetylase inhibitor valproic acid. Clin. Cancer Res. 14, 5410–5415 (2008).
Karagiannis, T. C., Kn, H. & El-Osta, A. The epigenetic modifier, valproic acid, enhances radiation sensitivity. Epigenetics 1, 131–137 (2006).
Noguchi, H. et al. Successful treatment of anaplastic thyroid carcinoma with a combination of oral valproic acid, chemotherapy, radiation and surgery. Endocr. J. 56, 245–249 (2009).
Ree, A. H. et al. Vorinostat, a histone deacetylase inhibitor, combined with pelvic palliative radiotherapy for gastrointestinal carcinoma: the Pelvic Radiation and Vorinostat (PRAVO) phase 1 study. Lancet Oncol. 11, 459–464 (2010).
Chen, H. P., Zhao, Y. T. & Zhao, T. C. Histone deacetylases and mechanisms of regulation of gene expression. Crit. Rev. Oncog. 20, 35–47 (2015).
Ren, J. et al. HDAC as a therapeutic target for treatment of endometrial cancers. Curr. Pharm. Des. 20, 1847–1856 (2014).
Nakajima, S. & Kitamura, M. Bidirectional regulation of NF-κB by reactive oxygen species: a role of unfolded protein response. Free Radic. Biol. Med. 65, 162–174 (2013).
Yoon, J. Y., Ishdorj, G., Graham, B. A., Johnston, J. B. & Gibson, S. B. Valproic acid enhances fludarabine-induced apoptosis mediated by ROS and involving decreased AKT and ATM activation in B-cell-lymphoid neoplastic cells. Apoptosis 19, 191–200 (2014).
Jeong, S. G. & Cho, G. W. Trichostatin a modulates intracellular reactive oxygen species through SOD2 and FOXO1 in human bone marrow-mesenchymal stem cells. Cell Biochem. Funct. 33, 37–43 (2014).
Park, S. et al. Suberoylanilide hydroxamic acid induces ROS-mediated cleavage of HSP90 in leukemia cells. Cell Stress Chaperones 20, 149–157 (2015).
Wolf, I. M. et al. Histone deacetylases inhibition by SAHA/vorinostat normalizes the glioma microenvironment via xCT equilibration. Sci. Rep. 4, 6226 (2014).
Sholler, G. S. et al. PCI-24781 (abexinostat), a novel histone deacetylase inhibitor, induces reactive oxygen species-dependent apoptosis and is synergistic with bortezomib in neuroblastoma. J. Cancer Ther. Res. 2, 21 (2013).
Bakkenist, C. J. & Kastan, M. B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499–506 (2003).
Wang, H. et al. The HDAC inhibitor depsipeptide transactivates the p53/p21 pathway by inducing DNA damage. DNA Repair (Amst.) 11, 146–156 (2012).
Blattmann, C. et al. Enhancement of radiation response in osteosarcoma and rhabdomyosarcoma cell lines by histone deacetylase inhibition. Int. J. Radiat. Oncol. Biol. Phys. 78, 237–245 (2010).
Kachhap, S. K. et al. Downregulation of homologous recombination DNA repair genes by HDAC inhibition in prostate cancer is mediated through the E2F1 transcription factor. PLoS ONE 5, e11208 (2010).
Ren, J. et al. Epigenetic interventions increase the radiation sensitivity of cancer cells. Curr. Pharm. Des. 20, 1857–1865 (2014).
Brown, S. L., Kolozsvary, A., Liu, J., Ryu, S. & Kim, J. H. Histone deacetylase inhibitors protect against and mitigate the lethality of total-body irradiation in mice. Radiat. Res. 169, 474–478 (2008).
Grabiec, A. M., Tak, P. P. & Reedquist, K. A. Function of histone deacetylase inhibitors in inflammation. Crit. Rev. Immunol. 31, 233–263 (2011).
Kim, H. J. & Chuang, D. M. HDAC inhibitors mitigate ischemia-induced oligodendrocyte damage: potential roles of oligodendrogenesis, VEGF, and anti-inflammation. Am. J. Transl. Res. 6, 206–223 (2014).
Felice, C., Lewis, A., Armuzzi, A., Lindsay, J. O. & Silver, A. Review article: selective histone deacetylase isoforms as potential therapeutic targets in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 41, 26–38 (2015).
Morris, Z. S. & Harari, P. M. Interaction of radiation therapy with molecular targeted agents. J. Clin. Oncol. 32, 2886–2893 (2014).
Chen, D. J. & Nirodi, C. S. The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clin. Cancer Res. 13, 6555–6560 (2007).
Kim, K. et al. Epidermal growth factor receptor vIII expression in U87 glioblastoma cells alters their proteasome composition, function, and response to irradiation. Mol. Cancer Res. 6, 426–434 (2008).
Baumann, M. et al. EGFR-targeted anti-cancer drugs in radiotherapy: preclinical evaluation of mechanisms. Radiother. Oncol. 83, 238–248 (2007).
Bonner, J. A. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006).
Bonner, J. A. et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 11, 21–28 (2010).
Ang, K. K. et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J. Clin. Oncol. 32, 2940–2950 (2014).
Debucquoy, A., Machiels, J. P., McBride, W. H. & Haustermans, K. Integration of epidermal growth factor receptor inhibitors with preoperative chemoradiation. Clin. Cancer Res. 16, 2709–2714 (2010).
Kriegs, M. et al. Radiosensitization of NSCLC cells by EGFR inhibition is the result of an enhanced p53-dependent G1 arrest. Radiother. Oncol. http://dx.doi.org/10.1016/j.radonc.2015.02.018 (2015).
Dent, P. et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat. Res. 159, 283–300 (2003).
Liccardi, G., Hartley, J. A. & Hochhauser, D. EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res. 71, 1103–1114 (2011).
Knebel, A., Rahmsdorf, H. J., Ullrich, A. & Herrlich, P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 15, 5314–5325 (1996).
Schmidt-Ullrich, R. K. et al. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene 15, 1191–1197 (1997).
Kim, J., Adam, R. M. & Freeman, M. R. Trafficking of nuclear heparin-binding epidermal growth factor-like growth factor into an epidermal growth factor receptor-dependent autocrine loop in response to oxidative stress. Cancer Res. 65, 8242–8249 (2005).
Kefaloyianni, E., Gaitanaki, C. & Beis, I. ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-κB transactivation during oxidative stress in skeletal myoblasts. Cell Signal. 18, 2238–2251 (2006).
Papaiahgari, S., Zhang, Q., Kleeberger, S. R., Cho, H. Y. & Reddy, S. P. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS–EGFR–PI3K–Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid. Redox Signal. 8, 43–52 (2006).
Han, W. & Lo, H. W. Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett. 318, 124–134 (2012).
Dittmann, K. et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J. Biol. Chem. 280, 31182–31189 (2005).
Dittmann, K., Mayer, C. & Rodemann, H. P. Nuclear EGFR as novel therapeutic target: insights into nuclear translocation and function. Strahlenther. Onkol. 186, 1–6 (2010).
Liccardi, G., Hartley, J. A. & Hochhauser, D. Importance of EGFR/ERCC1 interaction following radiation-induced DNA damage. Clin. Cancer Res. 20, 3496–3506 (2014).
Javvadi, P. et al. Threonine 2609 phosphorylation of the DNA-dependent protein kinase is a critical prerequisite for epidermal growth factor receptor-mediated radiation resistance. Mol. Cancer Res. 10, 1359–1368 (2012).
Kim, I. A. et al. Epigenetic modulation of radiation response in human cancer cells with activated EGFR or HER-2 signaling: potential role of histone deacetylase 6. Radiother. Oncol. 92, 125–132 (2009).
Dittmann, K., Mayer, C., Rodemann, H. P. & Huber, S. M. EGFR cooperates with glucose transporter SGLT1 to enable chromatin remodeling in response to ionizing radiation. Radiother. Oncol. 107, 247–251 (2013).
Dittmann, K. et al. Nuclear epidermal growth factor receptor modulates cellular radio-sensitivity by regulation of chromatin access. Radiother. Oncol. 99, 317–322 (2011).
Vlashi, E., McBride, W. H. & Pajonk, F. Radiation responses of cancer stem cells. J. Cell Biochem. 108, 339–342 (2009).
Vlashi, E. & Pajonk, F. Targeted cancer stem cell therapies start with proper identification of the target. Mol. Cancer Res. 8, 291 (2010).
Phillips, T. M., McBride, W. H. & Pajonk, F. The response of CD24−/low/CD44+ breast cancer-initiating cells to radiation. J. Natl Cancer Inst. 98, 1777–1785 (2006).
Bao, S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006).
Woodward, W. A. et al. WNT/β-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc. Natl Acad. Sci. USA 104, 618–623 (2007).
Kim, Y., Joo, K. M., Jin, J. & Nam, D. H. Cancer stem cells and their mechanism of chemo-radiation resistance. Int. J. Stem Cells 2, 109–114 (2009).
Rich, J. N. Cancer stem cells in radiation resistance. Cancer Res. 67, 8980–8984 (2007).
Bourguignon, L. Y., Shiina, M. & Li, J. J. Hyaluronan–CD44 interaction promotes oncogenic signaling, microRNA functions, chemoresistance, and radiation resistance in cancer stem cells leading to tumor progression. Adv. Cancer Res. 123, 255–275 (2014).
McCord, A. M., Jamal, M., Williams, E. S., Camphausen, K. & Tofilon, P. J. CD133+ glioblastoma stem-like cells are radiosensitive with a defective DNA damage response compared with established cell lines. Clin. Cancer Res. 15, 5145–5153 (2009).
Zielske, S. P., Spalding, A. C., Wicha, M. S. & Lawrence, T. S. Ablation of breast cancer stem cells with radiation. Transl. Oncol. 4, 227–233 (2011).
Vlashi, E. et al. Metabolic state of glioma stem cells and nontumorigenic cells. Proc. Natl Acad. Sci. USA 108, 16062–16067 (2011).
Diehn, M. et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458, 780–783 (2009).
Zafarana, G. & Bristow, R. G. Tumor senescence and radioresistant tumor-initiating cells (TICs): let sleeping dogs lie! Breast Cancer Res. 12, 111 (2010).
Dong, Q. et al. Radioprotective effects of BMI-1 involve epigenetic silencing of oxidase genes and enhanced DNA repair in normal human keratinocytes. J. Invest. Dermatol. 131, 1216–1225 (2011).
Pignalosa, D. & Durante, M. Overcoming resistance of cancer stem cells. Lancet Oncol. 13, e187–e188 (2012).
Chen, T. et al. Effects of heterochromatin in colorectal cancer stem cells on radiosensitivity. Chin. J Cancer 29, 270–276 (2010).
Vlashi, E. & Pajonk, F. Cancer stem cells, cancer cell plasticity and radiation therapy. Semin. Cancer Biol. 31, 28–35 (2015).
Lagadec, C., Vlashi, E., Della Donna, L., Dekmezian, C. & Pajonk, F. Radiation-induced reprogramming of breast cancer cells. Stem Cells 30, 833–844 (2012).
Shoshani, O. & Zipori, D. Stress as a fundamental theme in cell plasticity. Biochim. Biophys. Acta 1849, 371–377 (2015).
Chlon, T. M. et al. High-risk human papillomavirus E6 protein promotes reprogramming of Fanconi anemia patient cells through repression of p53 but does not allow for sustained growth of induced pluripotent stem cells. J. Virol. 88, 11315–11326 (2014).
Gomez-Casal, R. et al. Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol. Cancer 12, 94 (2013).
Patel, S. S., Shah, K. A., Shah, M. J., Kothari, K. C. & Rawal, R. M. Cancer stem cells and stemness markers in oral squamous cell carcinomas. Asian Pac. J. Cancer Prev. 15, 8549–8556 (2014).
Venere, M. et al. Therapeutic targeting of constitutive PARP activation compromises stem cell phenotype and survival of glioblastoma-initiating cells. Cell Death Differ. 21, 258–269 (2014).
Gilabert, M. et al. Poly(ADP-ribose) polymerase 1 (PARP1) overexpression in human breast cancer stem cells and resistance to olaparib. PLoS ONE 9, e104302 (2014).
Yoshikawa, M. et al. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 73, 1855–1866 (2013).
Greenow, K. & Clarke, A. R. Controlling the stem cell compartment and regeneration in vivo: the role of pluripotency pathways. Physiol. Rev. 92, 75–99 (2012).
Takebe, N. et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat. Rev. Clin. Oncol. http://dx.doi.org/10.1038/nrclinonc.2015.61 (2015).
Mizugaki, H. et al. γ-secretase inhibitor enhances antitumour effect of radiation in Notch-expressing lung cancer. Br. J. Cancer 106, 1953–1959 (2012).
Lin, J., Zhang, X. M., Yang, J. C., Ye, Y. B. & Luo, S. Q. γ-secretase inhibitor-I enhances radiosensitivity of glioblastoma cell lines by depleting CD133+ tumor cells. Arch. Med. Res. 41, 519–529 (2010).
Lagadec, C. et al. Radiation-induced Notch signaling in breast cancer stem cells. Int. J. Radiat. Oncol. Biol. Phys. 87, 609–618 (2013).
Diaz-Padilla, I. et al. A phase Ib combination study of RO4929097, a γ-secretase inhibitor, and temsirolimus in patients with advanced solid tumors. Invest. New Drugs 31, 1182–1191 (2013).
De Strooper, B. & Chavez Gutierrez, L. Learning by failing: ideas and concepts to tackle γ-secretases in Alzheimer disease and beyond. Annu. Rev. Pharmacol. Toxicol. 55, 419–437 (2015).
Kim, S. H., Kim, J. H. & Fried, J. Enhancement of the radiation response of cultured tumor cells by chloroquine. Cancer 32, 536–540 (1973).
Firat, E., Weyerbrock, A., Gaedicke, S., Grosu, A. L. & Niedermann, G. Chloroquine or chloroquine–PI3K/Akt pathway inhibitor combinations strongly promote gamma-irradiation-induced cell death in primary stem-like glioma cells. PLoS ONE 7, e47357 (2012).
Maycotte, P. & Thorburn, A. Targeting autophagy in breast cancer. World J. Clin. Oncol. 5, 224–240 (2014).
Ratikan, J. A., Sayre, J. W. & Schaue, D. Chloroquine engages the immune system to eradicate irradiated breast tumors in mice. Int. J. Radiat. Oncol. Biol. Phys. 87, 761–768 (2013).
Balic, A. et al. Chloroquine targets pancreatic cancer stem cells via inhibition of CXCR4 and hedgehog signaling. Mol. Cancer Ther. 13, 1758–1771 (2014).
Xiao, W. et al. CD44 is a biomarker associated with human prostate cancer radiation sensitivity. Clin. Exp. Metastasis 29, 1–9 (2012).
Ni, J. et al. CD44 variant 6 is associated with prostate cancer metastasis and chemo-/radioresistance. Prostate 74, 602–617 (2014).
Timmerman, L. A. et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 24, 450–465 (2013).
Wang, F. & Yang, Y. Suppression of the xCT–CD44v antiporter system sensitizes triple-negative breast cancer cells to doxorubicin. Breast Cancer Res. Treat. 147, 203–210 (2014).
Takeuchi, S. et al. Sulfasalazine and temozolomide with radiation therapy for newly diagnosed glioblastoma. Neurol. India 62, 42–47 (2014).
McDonald, J. T. et al. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 70, 8886–8895 (2010).
Bauer, A. K., Hill, T. 3rd & Alexander, C. M. The involvement of NRF2 in lung cancer. Oxid. Med. Cell Longev. 2013, 746432 (2013).
Hast, B. E. et al. Cancer-derived mutations in KEAP1 impair NRF2 degradation but not ubiquitination. Cancer Res. 74, 808–817 (2014).
Tao, S. et al. Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Res. 74, 7430–7441 (2014).
Reuter, S., Gupta, S. C., Chaturvedi, M. M. & Aggarwal, B. B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 49, 1603–1616 (2010).
Hayes, J. D. & Dinkova-Kostova, A. T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 39, 199–218 (2014).
Buettner, G. R., Wagner, B. A. & Rodgers, V. G. Quantitative redox biology: an approach to understand the role of reactive species in defining the cellular redox environment. Cell Biochem. Biophys. 67, 477–483 (2013).
Ma, T. et al. Dual-functional probes for sequential thiol and redox homeostasis sensing in live cells. Analyst 140, 322–329 (2015).
Kruger, A. & Ralser, M. ATM is a redox sensor linking genome stability and carbon metabolism. Sci. Signal. 4, pe17 (2011).
Perry, J. J. & Tainer, J. A. All stressed out without ATM kinase. Sci. Signal. 4, pe18 (2011).
Shirwany, N. A. & Zou, M. H. AMPK: a cellular metabolic and redox sensor. A minireview. Front. Biosci. (Landmark Ed.) 19, 447–474 (2014).
McBride, W. H. et al. A sense of danger from radiation. Radiat. Res. 162, 1–19 (2004).
Vazquez-Martin, A. et al. Repositioning chloroquine and metformin to eliminate cancer stem cell traits in pre-malignant lesions. Drug Resist. Updat. 14, 212–223 (2011).
Najbauer, J., Kraljik, N. & Nemeth, P. Glioma stem cells: markers, hallmarks and therapeutic targeting by metformin. Pathol. Oncol. Res. 20, 789–797 (2014).
Skinner, H. D. et al. Metformin use and improved response to therapy in rectal cancer. Cancer Med. 2, 99–107 (2013).
Spratt, D. E. et al. Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur. Urol. 63, 709–716 (2013).
Skinner, H. D. et al. Metformin use and improved response to therapy in esophageal adenocarcinoma. Acta Oncol. 52, 1002–1009 (2013).
Ferro, A. et al. Evaluation of diabetic patients with breast cancer treated with metformin during adjuvant radiotherapy. Int. J. Breast Cancer 2013, 659723 (2013).
Eckers, J. C., Kalen, A. L., Xiao, W., Sarsour, E. H. & Goswami, P. C. Selenoprotein P inhibits radiation-induced late reactive oxygen species accumulation and normal cell injury. Int. J. Radiat. Oncol. Biol. Phys. 87, 619–625 (2013).
Gao, Z. et al. Late ROS accumulation and radiosensitivity in SOD1-overexpressing human glioma cells. Free Radic. Biol. Med. 45, 1501–1509 (2008).
Le, O. N. et al. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell 9, 398–409 (2010).
Sabin, R. J. & Anderson, R. M. Cellular Senescence—its role in cancer and the response to ionizing radiation. Genome Integr. 2, 7 (2011).
Rodier, F. et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J. Cell Sci. 124, 68–81 (2011).
Durante, M., Reppingen, N. & Held, K. D. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol. Med. 19, 565–582 (2013).
Coppe, J. P. et al. Tumor suppressor and aging biomarker p16INK4a induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 286, 36396–36403 (2011).
Westbrook, A. M. et al. The role of tumour necrosis factor-alpha and tumour necrosis factor receptor signalling in inflammation-associated systemic genotoxicity. Mutagenesis 27, 77–86 (2012).
Schaue, D. & McBride, W. H. Links between innate immunity and normal tissue radiobiology. Radiation Res. 173, 406–417 (2010).
Kansara, M. et al. Immune response to RB1-regulated senescence limits radiation-induced osteosarcoma formation. J. Clin. Invest. 123, 5351–5360 (2013).
Klammer, H., Mladenov, E., Li, F. & Iliakis, G. Bystander effects as manifestation of intercellular communication of DNA damage and of the cellular oxidative status. Cancer Lett. 356, 58–71 (2015).
Kim, K. et al. High throughput screening of small molecule libraries for modifiers of radiation responses. Int. J. Radiat. Biol. 87, 839–845 (2011).
Demaria, S., Bhardwaj, N., McBride, W. H. & Formenti, S. C. Combining radiotherapy and immunotherapy: a revived partnership. Int. J. Radiat. Oncol. Biol. Phys. 63, 655–666 (2005).
Formenti, S. C. & Demaria, S. Radiation therapy to convert the tumor into an in situ vaccine. Int. J. Radiat. Oncol. Biol. Phys. 84, 879–880 (2012).
Schaue, D., Ratikan, J. A., Iwamoto, K. S. & McBride, W. H. Maximizing tumor immunity with fractionated radiation. Int. J. Radiat. Oncol. Biol. Phys. 83, 1306–1310 (2012).
Schaue, D. et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin. Cancer Res. 14, 4883–4890 (2008).
Liao, Y.-P., Meng, W. S. & McBride, W. H. Antigen presentation by dendritic cells is affected after irradiation [abstract]. Proc. Am. Assoc. Cancer Res. 43, 480–481 (2002).
Dewan, M. Z. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Butterfield, L. H. et al. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin. Cancer Res. 9, 998–1008 (2003).
Llosa, N. J. et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 5, 43–51 (2015).
Mullen, C. A., Coale, M. M., Lowe, R. & Blaese, R. M. Tumors expressing the cytosine deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild type tumor. Cancer Res. 54, 1503–1506 (1994).
Pizova, K. et al. Photodynamic therapy for enhancing antitumour immunity. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 156, 93–102 (2012).
Chen, F. H. et al. Radiotherapy decreases vascular density and causes hypoxia with macrophage aggregation in TRAMP-C1 prostate tumors. Clin. Cancer Res. 15, 1721–1729 (2009).
McBride, W. H. Phenotype and functions of intratumoral macrophages. Biochim. Biophys. Acta 865, 27–41 (1986).
Gajewski, T. F. et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr. Opin. Immunol. 25, 268–276 (2013).
Dougherty, G. J. & McBride, W. H. Immunoregulating activity of tumor-associated macrophages. Cancer Immunol. Immunother. 23, 67–72 (1986).
Kikuchi, N. et al. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and TH1/TH2 balance. Respir. Res. 11, 31 (2010).
Rockwell, C. E., Zhang, M., Fields, P. E. & Klaassen, C. D. TH2 skewing by activation of Nrf2 in CD4+ T cells. J. Immunol. 188, 1630–1637 (2012).
Schaue, D., Xie, M. W., Ratikan, J. A. & McBride, W. H. Regulatory T cells in radiotherapeutic responses. Front. Oncol. 2, 90 (2012).
Tsai, C. S. et al. Macrophages from irradiated tumors express higher levels of iNOS, arginase-1 and COX-2, and promote tumor growth. Int. J. Radiat. Oncol. Biol. Phys. 68, 499–507 (2007).
Kioi, M. et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Invest. 120, 694–705 (2010).
Tseng, D., Vasquez-Medrano, D. A. & Brown, J. M. Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas. Br. J. Cancer 104, 1805–1809 (2011).
Chen, F. H. et al. Combination of vessel-targeting agents and fractionated radiation therapy: the role of the SDF-1/CXCR4 pathway. Int. J. Radiat. Oncol. Biol. Phys. 86, 777–784 (2013).
Brown, J. M. Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy. Br. J. Radiol. 87, 20130686 (2014).
Xu, J. et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 73, 2782–2794 (2013).
Chiang, C. S. et al. Irradiation promotes an M2 macrophage phenotype in tumor hypoxia. Front. Oncol. 2, 89 (2012).
Ahn, G. O. & Brown, J. M. Influence of bone marrow-derived hematopoietic cells on the tumor response to radiotherapy: experimental models and clinical perspectives. Cell Cycle 8, 970–976 (2009).
Mantovani, A. & Allavena, P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 212, 435–445 (2015).
Tsai, J. H. et al. Ionizing radiation inhibits tumor neovascularization by inducing ineffective angiogenesis. Cancer Biol. Ther. 4, 1395–1400 (2005).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Johnson, D. B., Rioth, M. J. & Horn, L. Immune checkpoint inhibitors in NSCLC. Curr. Treat. Options Oncol. 15, 658–669 (2014).
Demaria, S. et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734 (2005).
Pilones, K. A., Vanpouille-Box, C. & Demaria, S. Combination of radiotherapy and immune checkpoint inhibitors. Semin. Radiat. Oncol. 25, 28–33 (2015).
Deng, L. et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 124, 687–695 (2014).
Stamell, E. F., Wolchok, J. D., Gnjatic, S., Lee, N. Y. & Brownell, I. The abscopal effect associated with a systemic anti-melanoma immune response. Int. J. Radiat. Oncol. Biol. Phys. 85, 293–295 (2013).
Hiniker, S. M. et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl. Oncol. 5, 404–407 (2012).
Postow, M. A., Harding, J. & Wolchok, J. D. Targeting immune checkpoints: releasing the restraints on anti-tumor immunity for patients with melanoma. Cancer J. 18, 153–159 (2012).
Acknowledgements
The authors would like to dedicate this article to H. Rodney Withers, past Chair of Radiation Oncology at the University of California, Los Angeles (UCLA), who died on 25 February 2015. 'Rod' was an intellectual giant who shaped the discipline of radiobiology as it related to radiotherapy. He is greatly missed. The authors would also like to thank Dr Ekaterini Angelis for editorial assistance. Financial support for the authors came from the US Army MTA W81XWH-11-1-0531 (W.H.M.) and NIAID 2U19 AI067769 (W.H.M.).
Author information
Authors and Affiliations
Contributions
Both the authors researched data for article, contributed to discussion of the content, wrote the article, and reviewed and edited the manuscript before submission and following peer review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Schaue, D., McBride, W. Opportunities and challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol 12, 527–540 (2015). https://doi.org/10.1038/nrclinonc.2015.120
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2015.120
This article is cited by
-
CYLD induces high oxidative stress and DNA damage through class I HDACs to promote radiosensitivity in nasopharyngeal carcinoma
Cell Death & Disease (2024)
-
Sarcoma cell-specific radiation sensitization by titanate scrolled nanosheets: insights from physicochemical analysis and transcriptomic profiling
Scientific Reports (2024)
-
Radiotherapy combined with nano-biomaterials for cancer radio-immunotherapy
Journal of Nanobiotechnology (2023)
-
APE1 promotes non-homologous end joining by initiating DNA double-strand break formation and decreasing ubiquitination of artemis following oxidative genotoxic stress
Journal of Translational Medicine (2023)
-
Targeting MHC-I molecules for cancer: function, mechanism, and therapeutic prospects
Molecular Cancer (2023)