Abstract
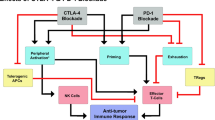
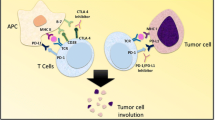
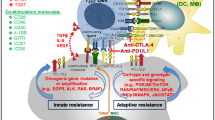
Immune checkpoint inhibitors (ICIs)—such as antibodies to programmed cell death–1 (PD-1), to its ligand PD-L1, or to cytotoxic T lymphocyte-associated protein–4 (CTLA-4)—are an evolving treatment option for several types of cancer, but only a limited number of patients benefit from such therapy. Preclinical studies have suggested that the combination of PD-1 or PD-L1 inhibitors with either cytotoxic chemotherapy or antibodies to CTLA-4 is a promising treatment strategy for advanced cancer. Indeed, combinations of cytotoxic chemotherapy and PD-1/PD-L1 inhibitors have been approved and are now used in clinical practice for the treatment of advanced non-small cell lung cancer and small cell lung cancer on the basis of positive results of large-scale clinical trials. In addition, the combination of antibodies to CTLA-4 (ipilimumab) and to PD-1 (nivolumab) has been found to confer a survival benefit in patients with melanoma or renal cell carcinoma. Several ongoing clinical trials are also investigating ICI combination therapy in comparison with standard therapy for other tumor types. The identification of patients likely to achieve a sufficient benefit from PD-1/PD-L1 inhibitor monotherapy remains a challenge; however, with the establishment of novel complementary biomarkers being needed. Preclinical and clinical investigations of immune-related adverse events of ICI combination therapy are also warranted to establish management strategies. In this review, we summarize the current landscape of combination therapy with PD-1/PD-L1 inhibitors plus either cytotoxic chemotherapy or CTLA-4 inhibitors to clarify the benefits of and outstanding clinical issues related to such treatment.
Similar content being viewed by others
References
Ishida Y, Agata Y, Shibahara K et al (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11(11):3887–3895
Freeman GJ, Long AJ, Iwai Y et al (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192(7):1027–1034
Latchman Y, Wood CR, Chernova T et al (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2(3):261–268. https://doi.org/10.1038/85330
Okazaki T, Chikuma S, Iwai Y et al (2013) A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 14(12):1212–1218. https://doi.org/10.1038/ni.2762
Galon J, Bruni D (2019) Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 18(3):197–218. https://doi.org/10.1038/s41573-018-0007-y
Galluzzi L, Senovilla L, Zitvogel L et al (2012) The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 11(3):215–233. https://doi.org/10.1038/nrd3626
Kepp O, Galluzzi L, Martins I et al (2011) Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metast Rev 30(1):61–69. https://doi.org/10.1007/s10555-011-9273-4
Krysko DV, Garg AD, Kaczmarek A et al (2012) Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 12(12):860–875. https://doi.org/10.1038/nrc3380
Obeid M, Tesniere A, Ghiringhelli F et al (2007) Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13(1):54–61. https://doi.org/10.1038/nm1523
Apetoh L, Ghiringhelli F, Tesniere A et al (2007) Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13(9):1050–1059. https://doi.org/10.1038/nm1622
Ghiringhelli F, Apetoh L, Tesniere A et al (2009) Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 15(10):1170–1178. https://doi.org/10.1038/nm.2028
Michaud M, Martins I, Sukkurwala AQ et al (2011) Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334(6062):1573–1577. https://doi.org/10.1126/science.1208347
Wang Z, Till B, Gao Q (2017) Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. Oncoimmunology 6(7):e1331807. https://doi.org/10.1080/2162402X.2017.1331807
Park JH, Jang M, Tarhan YE et al (2016) Clonal expansion of antitumor T cells in breast cancer correlates with response to neoadjuvant chemotherapy. Int J Oncol 49(2):471–478. https://doi.org/10.3892/ijo.2016.3540
de Biasi AR, Villena-Vargas J, Adusumilli PS (2014) Cisplatin-induced antitumor immunomodulation: a review of preclinical and clinical evidence. Clin Cancer Res 20(21):5384–5391. https://doi.org/10.1158/1078-0432.CCR-14-1298
Roselli M, Cereda V, di Bari MG et al (2013) Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology 2(10):e27025. https://doi.org/10.4161/onci.27025
Sevko A, Michels T, Vrohlings M et al (2013) Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol 190(5):2464–2471. https://doi.org/10.4049/jimmunol.1202781
Brahmer J, Reckamp KL, Baas P et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135. https://doi.org/10.1056/NEJMoa1504627
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639. https://doi.org/10.1056/NEJMoa1507643
Herbst RS, Baas P, Kim DW et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389(10066):255–265. https://doi.org/10.1016/S0140-6736(16)32517-X
Reck M, Rodriguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833. https://doi.org/10.1056/NEJMoa1606774
Rizvi NA, Hellmann MD, Brahmer JR et al (2016) Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 34(25):2969–2979. https://doi.org/10.1200/JCO.2016.66.9861
Kanda S, Goto K, Shiraishi H et al (2016) Safety and efficacy of nivolumab and standard chemotherapy drug combination in patients with advanced non-small-cell lung cancer: a four arms phase Ib study. Ann Oncol 27(12):2242–2250. https://doi.org/10.1093/annonc/mdw416
Gandhi L, Rodriguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092. https://doi.org/10.1056/NEJMoa1801005
Paz-Ares L, Luft A, Vicente D et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379(21):2040–2051. https://doi.org/10.1056/NEJMoa1810865
Burtness B, Bratland Å, Fuereder T et al (2018) LBA8_PRKEYNOTE-048: phase III study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). Ann Oncol. https://doi.org/10.1093/annonc/mdy424.045
Socinski MA, Jotte RM, Cappuzzo F et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301. https://doi.org/10.1056/NEJMoa1716948
Reck M, Mok TSK, Nishio M et al (2019) Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. https://doi.org/10.1016/S2213-2600(19)30084-0
Gainor JF, Shaw AT, Sequist LV et al (2016) EGFR mutations and alk rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 22(18):4585–4593. https://doi.org/10.1158/1078-0432.CCR-15-3101
Haratani K, Hayashi H, Tanaka T et al (2017) Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol 28(7):1532–1539. https://doi.org/10.1093/annonc/mdx183
Spigel DR, Schrock AB, Fabrizio D et al (2016) Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J Clin Oncol 34(15_suppl):9017. https://doi.org/10.1200/JCO.2016.34.15_suppl.9017
Horn L, Mansfield AS, Szczesna A et al (2018) First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 379(23):2220–2229. https://doi.org/10.1056/NEJMoa1809064
Alexandrov LB, Nik-Zainal S, Wedge DC et al (2013) Signatures of mutational processes in human cancer. Nature 500(7463):415–421. https://doi.org/10.1038/nature12477
Yu H, Batenchuk C, Badzio A et al (2017) PD-L1 expression by two complementary diagnostic assays and mrna in situ hybridization in small cell lung cancer. J Thorac Oncol 12(1):110–120. https://doi.org/10.1016/j.jtho.2016.09.002
Carvajal-Hausdorf D, Altan M, Velcheti V et al (2019) Expression and clinical significance of PD-L1, B7–H3, B7–H4 and TILs in human small cell lung Cancer (SCLC). J Immunother Cancer 7(1):65. https://doi.org/10.1186/s40425-019-0540-1
Langer CJ, Gadgeel SM, Borghaei H et al (2016) Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 17(11):1497–1508. https://doi.org/10.1016/S1470-2045(16)30498-3
Borghaei H, Langer CJ, Gadgeel S et al (2019) 24-month overall survival from KEYNOTE-021 Cohort G: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol 14(1):124–129. https://doi.org/10.1016/j.jtho.2018.08.004
Zinner RG, Obasaju CK, Spigel DR et al (2015) PRONOUNCE: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients ith advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol 10(1):134–142. https://doi.org/10.1097/JTO.0000000000000366
Patel JD, Hensing TA, Rademaker A et al (2009) Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol 27(20):3284–3289. https://doi.org/10.1200/JCO.2008.20.8181
Rodrigues-Pereira J, Kim JH, Magallanes M et al (2011) A randomized phase 3 trial comparing pemetrexed/carboplatin and docetaxel/carboplatin as first-line treatment for advanced, nonsquamous non-small cell lung cancer. J Thorac Oncol 6(11):1907–1914. https://doi.org/10.1097/JTO.0b013e318226b5fa
Patel JD, Socinski MA, Garon EB et al (2013) PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol 31(34):4349–4357. https://doi.org/10.1200/JCO.2012.47.9626
Okamoto I, Aoe K, Kato T et al (2013) Pemetrexed and carboplatin followed by pemetrexed maintenance therapy in chemo-naive patients with advanced nonsquamous non-small-cell lung cancer. Invest New Drugs 31(5):1275–1282. https://doi.org/10.1007/s10637-013-9941-z
Gandhi L, Rodriguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. https://doi.org/10.1056/NEJMoa1801005
Weber JS, Hodi FS, Wolchok JD et al (2017) safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 35(7):785–792. https://doi.org/10.1200/JCO.2015.66.1389
Nakatani Y, Kawakami H, Ichikawa M et al (2018) Nivolumab-induced acute granulomatous tubulointerstitial nephritis in a patient with gastric cancer. Invest New Drugs 36(4):726–731. https://doi.org/10.1007/s10637-018-0596-7
Sanlorenzo M, Vujic I, Daud A et al (2015) Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 151(11):1206–1212. https://doi.org/10.1001/jamadermatol.2015.1916
Nakamura Y, Tanaka R, Asami Y et al (2017) Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol 44(2):117–122. https://doi.org/10.1111/1346-8138.13520
Hua C, Boussemart L, Mateus C et al (2016) Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 152(1):45–51. https://doi.org/10.1001/jamadermatol.2015.2707
Freeman-Keller M, Kim Y, Cronin H et al (2016) Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 22(4):886–894. https://doi.org/10.1158/1078-0432.CCR-15-1136
Teulings HE, Limpens J, Jansen SN et al (2015) Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 33(7):773–781. https://doi.org/10.1200/JCO.2014.57.4756
Haratani K, Hayashi H, Chiba Y et al (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4(3):374–378. https://doi.org/10.1001/jamaoncol.2017.2925
Teraoka S, Fujimoto D, Morimoto T et al (2017) Early Immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol 12(12):1798–1805. https://doi.org/10.1016/j.jtho.2017.08.022
Sato K, Akamatsu H, Murakami E et al (2018) Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 115:71–74. https://doi.org/10.1016/j.lungcan.2017.11.019
Kimbara S, Fujiwara Y, Iwama S et al (2018) Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci 109(11):3583–3590. https://doi.org/10.1111/cas.13800
Collins AV, Brodie DW, Gilbert RJ et al (2002) The interaction properties of costimulatory molecules revisited. Immunity 17(2):201–210
Walker LS, Sansom DM (2011) The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol 11(12):852–863. https://doi.org/10.1038/nri3108
Egen JG, Kuhns MS, Allison JP (2002) CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 3(7):611–618. https://doi.org/10.1038/ni0702-611
Takahashi T, Tagami T, Yamazaki S et al (2000) Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med 192(2):303–310
Melero I, Hervas-Stubbs S, Glennie M et al (2007) Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer 7(2):95–106. https://doi.org/10.1038/nrc2051
Warner AB, Postow MA (2018) Combination controversies: checkpoint inhibition alone or in combination for the treatment of melanoma? Oncology (Williston Park) 32(5):228–234
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373(1):23–34. https://doi.org/10.1056/NEJMoa1504030
Wolchok JD, Chiarion-Sileni V, Gonzalez R et al (2017) Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377(14):1345–1356. https://doi.org/10.1056/NEJMoa1709684
Motzer RJ, Tannir NM, McDermott DF et al (2018) Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378(14):1277–1290. https://doi.org/10.1056/NEJMoa1712126
Namikawa K, Kiyohara Y, Takenouchi T et al (2018) Efficacy and safety of nivolumab in combination with ipilimumab in Japanese patients with advanced melanoma: an open-label, single-arm, multicentre phase II study. Eur J Cancer 105:114–126. https://doi.org/10.1016/j.ejca.2018.09.025
Hellmann MD, Ciuleanu TE, Pluzanski A et al (2018) Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 378(22):2093–2104. https://doi.org/10.1056/NEJMoa1801946
Goodman AM, Kato S, Bazhenova L et al (2017) Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 16(11):2598–2608. https://doi.org/10.1158/1535-7163.MCT-17-0386
Rizvi NA, Hellmann MD, Snyder A et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348(6230):124–128. https://doi.org/10.1126/science.aaa1348
Rizvi H, Sanchez-Vega F, La K et al (2018) Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol 36(7):633–641. https://doi.org/10.1200/JCO.2017.75.3384
Gandara DR, Paul SM, Kowanetz M et al (2018) Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 24(9):1441–1448. https://doi.org/10.1038/s41591-018-0134-3
Schadendorf D, Hodi FS, Robert C et al (2015) Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 33(17):1889–1894. https://doi.org/10.1200/JCO.2014.56.2736
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H. Hayashi has received honoraria from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., and Taiho Pharmaceutical Co. Ltd. as well as research funding from AbbVie Inc., AC MEDICAL Inc., Astellas Pharma Inc., AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb Co. Ltd., Daiichi Sankyo Co. Ltd., Eisai Co. Ltd., Eli Lilly Japan K.K., EPS Associates Co. Ltd., GlaxoSmithKline K.K., Japan Clinical Research Operations Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Merck Serono Co. Ltd., MSD K.K., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., PAREXEL International Corp., Pfizer Japan Inc., PPD-SNBL K.K., Quintiles Transnational Japan K.K., Taiho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., and Yakult Honsha Co. Ltd. Y. Nakagawa has received honoraria from Astellas Pharma Inc., AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Clinical Trial Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Nichi-Iko Pharmaceutical Co. Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., Reno. Medical K.K., and Sym Bio Pharmaceuticals Ltd.; research funding from A2 Healthcare Corp., AbbVie Inc., Astellas Pharma Inc., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Eisai Co. Ltd., Eli Lilly Japan K.K., EP-CRSU Co. Ltd., GRITSTONE ONCOLOGY Inc., ICON Japan K.K., inVentiv Health Japan, MSD K.K., Linical Co. Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., PAREXEL International Corp., Pfizer Japan Inc., Quintiles, Taiho Pharmaceutical Co. Ltd., and Takeda Pharmaceutical Co. Ltd.; and consulting or advisory fees from Astellas Pharma Inc., Ono Pharmaceutical Co. Ltd., and Takeda Pharmaceutical Co. Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Hayashi, H., Nakagawa, K. Combination therapy with PD-1 or PD-L1 inhibitors for cancer. Int J Clin Oncol 25, 818–830 (2020). https://doi.org/10.1007/s10147-019-01548-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01548-1